Assessment of Phytochemicals, Proximate and Elemental Composition of Pterocarpus soyauxii (Oha) Leaves ()
1. Introduction
The culinary importance of most edible vegetables and plants consist basically of the vital nutrients which are required for healthy living of both animals and human beings which they possess. These vital nutrients which could be phytochemicals and useful mineral elements are necessary for curing certain ailments due to their medicinal properties. Pterocarpus soyauxii commonly called “Oha” by the Igbo speaking part of Nigeria is a popular green vegetable consumed in large quantities in the South Eastern part of Nigeria because of its unique taste and vitamin C content [1] [2] .
Dike [3] asserted that the crude extract of the leaves has been found to significantly increase red and white blood cells as well as haemoglobin of albino rats. The ground stem of this plant is also associated with aiding child birth and in the treatment of anaemic conditions [4] . In line with the above claims and much more, there is the need to assess the phytochemical, proximate and elemental compositions of Pterocarpus soyauxii to have sufficient scientific basis to verify or refute the claims of the several benefits accruing to this plant.
2. Materials and Methods
2.1. Sample Collection, Identification and Preparation
Fresh leaves of Pterocarpus soyauxii were collected from Ohianri forest Umuoti village, Mgbirichi in Ohaji-Egbema L.G.A, Imo State, Nigeria. Mr. P. O. Ugwuozor, a taxonomist attached to the Department of Botany, Nnamdi Azikiwe University, Awka, Nigeria identified the plant as Pterocarpus soyauxii. The leaves were destalked, air dried in the laboratory for twelve days and subsequently pulverized with mortar and pestle. The pulverized sample was kept in an air tight container for subsequent analysis.
2.2. Proximate Analysis
Standard methods [5] [6] were used to determine the moisture, ash, crude fat, crude protein, crude fibre and carbohydrate contents of the samples.
2.2.1. Moisture Content Analysis
The sample (1 g) was weighed into a petri dish, dried in the oven at 100˚C for 1 hour and weighed. The drying continued at 30 minute intervals until a constant weight was obtained. The final weight was recorded. The moisture content was calculated using the formula:
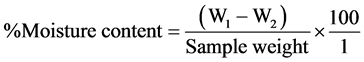
where: W1 = weight of petri dish + sample before drying;
W2 = weight of petri dish + sample after drying.
2.2.2. Ash Content Analysis
The sample (1 g) was weighed into a platinum crucible and placed on the Bunsen burner and heated until the sample turned to ash. It was cooled in a desiccator and weighed. The percentage ash content was calculated using the formula:
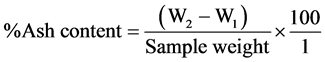
where: W1 = weight of empty platinum crucible;
W2 = weight of platinum crucible + ash sample.
2.2.3. Crude Fibre Analysis
Acid and base treatments were carried out as described below: 2.0 g of the sample was weighed into a 250 ml conical flask. 200 ml of 1.25% H2SO4 was added and the mixture heated for 30 minutes on a hot plate. It was then filtered and the residue washed with water until it was no more acidic. 200 ml of 1.25% NaOH was added to the residue in another 250 ml conical flask and the mixture heated for 30 minutes and filtered. The residue was dried in the oven, cooled in the desiccator and weighed. The residue was then transferred into a platinum crucible and heated on the Bunsen burner until it turned to ash. It was cooled in the desiccator and weighed again. Percentage crude fibre was calculated using the formula:
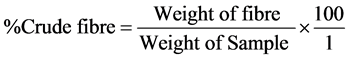
2.2.4. Protein Content Analysis
The sample (0.5 g) was weighed into a Kjeldahl flask. 1.0 g of CuSO4, 1.0 g of Na2SO4 and 20 ml of H2SO4 were added. The flask was heated on a heating mantle until the solution turned light green, showing complete digestion. The solution was allowed to stand for 24 hours, during which it solidified. After 24 hours, 200 ml of distilled water was used to dissolve the solidified mixture in the Kjeldahl flask. It was transferred to a 250 ml round bottomed flask and 60 ml of 40% NaOH and three pieces of zinc metal (catalyst) were added to the flask. The round bottomed flask was placed on a heating mantle and Kjeldahl distillation apparatus was mounted. To a 250 ml conical flask, 100 ml of 4% boric acid and two drops of screened methyl red indicator were added. The colour of the solution changed to pink. This setup was put at the absorber end of the apparatus. The solution in the round bottomed flask was heated and allowed to distill into the solution in the conical flask until the colour changed from pink to green. Thereafter, 20 ml of the solution was titrated with 0.1 M (NH4)2SO4 with screened methyl red as indicator, until the colour changed from green to pink. Percentage protein content was calculated using the formula:
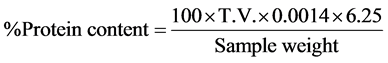
where: 0.0014 = a constant [quantity of protein liberated by 0.1 M (NH4)2SO4];
T.V. = titre value;
100 = conversion to percentage;
6.25 = protein constant according to Kjeldahl method.
2.2.5. Lipid Content Analysis
This was carried out using Soxhlet Extraction Apparatus. 10 g of the sample was put in a thimble. Extraction was carried out with n-hexane as the solvent. The n-hexane was distilled off and the oil was weighed. The percentage lipid content was calculated using the formula:
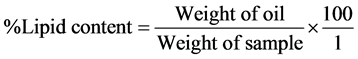
2.2.6. Carbohydrate Content Analysis
Percentage carbohydrate content was determined using the formula:
100 ? (% moisture + % ash + % protein + % fat + % crude fibre).
2.3. Estimation of Vitamins
2.3.1. Vitamin A
The sample (20 g) was weighed in a 250 ml conical flask. 50 ml of a mixture of acetone and low boiling petroleum ether (1:1 v/v) was added to the sample. The mixture was shaken for three hours using a shaker. It was cooled, filtered and trichloroacetic acid (2 ml) was added. The absorbance was obtained using UV/ Visible Spectrophotometer at 390 nm with acetone and diethylether blank. The concentration of vitamin A was calculated using the formula:
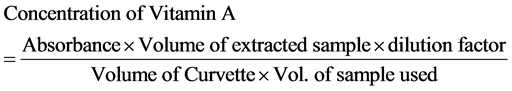
2.3.2. Vitamin C
The sample (20 g) was weighed into a 250 ml conical flask. 100 ml of 20% aqueous ethanol was poured into the sample and soaked for three minutes. It was shaken using a shaker for four hours and left overnight. The sample mixture was filtered with a whatman filter paper.
The filtrate (50 ml) was measured into a conical flask followed by the addition of 10 ml concentrated sulphuric acid and twenty drops of 1% starch indicator. The sample mixture was then titrated with 0.05 N iodine solution until a blue black colour was observed. The concentration of vitamin C was calculated using the formula:
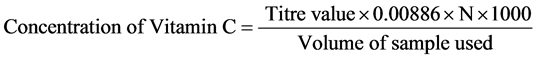
2.4. Elemental Analysis
The sample (0.2 g) was digested with 3 cm3 aqua regia (75 vol% hydrochloric acid and 25 vol% nitric acid) at a temperature of 100˚C. The digest was filtered into a 250 ml volumetric flask and made up to the mark with distilled water. The mixture was stirred and filtered using whatman filter paper. The filtrate was analyzed for Ca, Cu, Zn, Mg, K, Mn, Fe, Na, Pb and Cr using Atomic Absorption Spectrophotometer Varian AA 280. The results are shown in Table 1.
2.5. Phytochemical Screening
Phytochemical screening of the leaves extracts in five different solvents (distilled water, diethylether, ethanol, n-hexane and methanol) for the presence alkaloids, saponins, flavonoids, tannins, steroids, phenols and glycosides using cold maceration method were carried out [6] [7] . Each solvent (50 cm3) was used to extract the leaves separately. The results are shown in Table 2. The phytochemicals present were estimated quantitatively. The results are shown in Table 3.
![]()
Table 1. Element analysis of Pterocarpus soyauxii leaves.
![]()
Table 2.Qualitative phytochemical analysis of P.soyauxii leaves.
Key: + In significantly or slightly present. ++ Moderately present. +++ Abundantly present. − Absent.
![]()
Table 3. Quantitative analysis of the phytochemicals in Pterocarpus soyauxii (Oha) leaves.
2.5.1. Alkaloids
1 cm3 of 1% HCl was added to 3 cm3 of each extract in a test-tube. The mixture was heated for 20 minutes. It was cooled and filtered. The filtrate was used for the following tests:
2 drops of Mayers reagent was added to 1 cm3 of the extract. A creamy precipitate indicated the presence of alkaloids in the extract.
2 drops of Wagner’s reagent was added to 1 cm3 of the extract. A reddish brown precipitate indicated the presence of alkaloids in the extracts.
2.5.2. Saponins
Frothing Test: 2 cm3 of each extract in a test-tube was vigorously shaken for 2 minutes. Frothing indicated the presence of saponins in the extract.
Emulsion Test: 5 drops of olive oil was added to 3 cm3 of each extract in a test-tube and the mixture was vigorously shaken. Stable emulsion formed in each extract indicated the presence of saponins.
2.5.3. Flavonoids
1 cm3 of 10% NaOH was added to 3 cm3 of each extract. No yellow colouration was observed in all the extracts showing that flavonoids were absent in all the extracts.
2.5.4. Steroids
Salkowski Test: 5 drops of concentrated H2SO4 was added to 1 cm3 of each extract. No red colouration was observed in each extract tested. This result showed the absence of steroids in all the extracts.
2.5.5. Tannins
2 drops of 5% FeCl3 was added to 1 cm3 of each extract. A greenish precipitate indicated the presence of tannins in the extracts.
2.5.6. Test for Phenol
3 drops of 5% NaOH were added to 1 cm3 of each extract. Absence of an orange colour indicated the absence of phenol in all the extracts.
2.5.7. Glycosides
10 cm3 of 50% H2SO4 was added to 1 cm3 of each extract in a test-tube. The mixture was heated in boiling water for 15 minutes. 10 cm3 of Fehling’s solution was added and the mixture was boiled. A brick-red precipitate indicated the presence of glycosides.
3. Results and Discussion
3.1. Proximate Analysis
The result of proximate analysis in Table 4 showed the composition of moisture content, protein, ash content, crude fibre, lipids and carbohydrate of the leaves. The results showed that Pterocarpus soyauxii has moisture content of 17.2%. The value is higher than that earlier reported by Dike [3] . This may be due to the
![]()
Table 4. Proximate analysis of Pterocarpus soyauxii (Oha) leaf.
frequent rainfall in the area of study. The moisture content of any food is an index of its water activity and it is used as a measure of stability and susceptibility to microbial contamination. The high moisture content of the Pterocarpus soyauxii leaves explains its short shell life and could require extensive storage method.
The protein content of P. soyauxii leaves was 29.5%. The value of this study was higher than 15.40% reported by Dike [3] . The relatively high protein content suggests the high amount of essential amino acids which serve as an alternative source of energy when the carbohydrate metabolism is impaired via glucogenesis [8] . Plant protein still remains a major source of food nutrient for the less priviledged population in developing countries like Nigeria. Therefore, the protein content of the leaves of Pterocarpus soyauxii makes them suitable as a source of vegetable protein [9] .
Ash content (5.70%) was lower than the 7.14% obtained by Dike [3] . The ash of a food stuff is the inorganic residue remaining after the organic matter has been burnt away [10] . The ash content shows the leaves to be rich in mineral elements such as Na, Mg, Ca, Fe, etc.
Crude fibre content of the leaves (17.5%) was lower than the 18.98% reported by Dike [3] . The composition of leaves with high crude fibre content may contribute to a reduction in the incidence of certain diseases like colon cancer, coronary heart disease, diabetes, high blood pressure, obesity and other digestive disorder [11] .
The crude fat content of 4.15% of the leaves suggested that the leaves contain low quantities of lipid biomolecules [8] and cannot serve as main source of these biomolecules which are important for body metabolism.
The carbohydrate content (25.95%) is low when compared with 52.3% obtained by Dike [3] . These results do not agree with the suggestion that most vegetables are generally not good sources of carbohydrates [9] .
3.2. Vitamins
The estimate of vitamins A and C is shown in Table 5. The presence of vitamin A in the leaves make them good for the maintenance of immune system, good vision and also serves as an antioxidant.
The presence of vitamin C in this vegetable shows that the leaves could be used to promote healthy living such as maintenance of tissues, bones and teeth, protection against scurvy and other ascorbic acid deficiency related diseases [12] .
3.3. Elemental Analysis
The results of the elemental analysis of Pterocarpus soyauxii for the metals in
![]()
Table 5. Estimate of vitamins A and C in Pterocarpus soyauxii.
Table 1, show the following in mg/L: Ca (4.04), Cu (0.20), Zn (1.39), Mg (41.00), K (0.31), Fe (0.60), Mn (0.17) and Na (32.0). Cr and Pb were not present.
Calcium helps in bone formation and healthy teeth. It is also required for the metabolism of many enzymes and for blood coagulation [13] . Copper is involved in iron and lipid metabolism. It is also involved in connective tissue synthesis and maintenance of heart muscle. Deficiency of copper leads to impaired growth, degeneration of heart muscle, decrease in immune responses and may lead to increased incidences of infections [13] . Zinc forms zinc fingers (Zn2+ co- ordinate to four amino side chain) which provide structural stability to another 300 - 700 proteins and facilitates binding of protein to DNA. It is used therapeutically to promote wound healing and in treating gastric ulcers [14] . Magnesium is indispensible as a supplement in the metabolism of calcium, vitamin C, phosphorus, sodium and potassium. It is essential for proper nerve and muscle function effectively helping against stress and depression. Potassium regulates the activity of muscles and nerves because its cations are important in neuron function. It helps in the maintenance of the body’s pH level [15] . Sodium helps to maintain the balance of water, acids and bases in the fluid outside the cell. Iron is a component of the haemoglobin and mycoglobin. It is required for oxygen and carbon dioxide transport and oxidative phosphorylation [13] .
3.4. Phytochemical Analysis
Phytochemicals are natural bioactive compounds found in plants that work withnutrients and dietary fiber for disease protection [3] .
The results of phytochemical analysis in Table 2 show that alkaloids were present in the diethylether, ethanol and n-hexane extracts of the “Oha” leaves. They were absent in the distilled water and methanol extracts. The quantitative analysis of the phytochemicals in Table 3 shows that the composition of the alkaloids in the leaves was 2.66%. This was higher than the analysis earlier reported on the same species by Dike [3] . Dike reported a composition of 1.32%. Alkaloids have been used medicinally for the treatment of malaria, cold, cough, diabetes etc. [16] .
Glycosides were present in water, ethanol, n-hexane and methanol extracts of the leaves. They were absent in diethylether extract. The quantitative analysis of the phytochemicals in Table 3 shows the composition of glycosides to be 0.19%. The presence of glycosides in these leaves shows its medicinal property in the treatment of heart diseases.
Saponins were present in water, diethylether, ethanol and methanol extracts of the leaves and were absent in n-hexane extract. The composition of saponins was 1.2%. This was higher than that earlier reported (1.02%) by Dike [3] .
Tannins were present in ethanol and methanol extracts of the leaves. They were found to be absent in water, diethylether and n-hexane extracts. Table 3 showed that the composition of tannins in the leaves was 4.15%. The value was significantly higher than that reported (0.28%) by Dike [3] . Tannins are chelating agents for metals and can form complexes with macromolecules. Through this process, essential substrates and enzymes of micro-organisms are depleted leading to cell death.
Flavonoids, phenols and steroids were found to be absent in all the extracts.
4. Conclusion
Pterocarpus soyauxii (Oha) leaves serve as the indigenous vegetable for preparing tasty soup in the south-south and south-east part of Nigeria. They supplement the nutritive sources for animals and man. For example, the protein content is approximately close to the protein requirement of the body. The presence of many phytochemicals in the leaves helps to enrich the immune system and other haematological parameters in human body. The proximate composition, presence of many mineral elements and absence of heavy metals make the leaves very nutritive and safe.
5. Recommendation
Further studies should be carried out to isolate, characterize and elucidate the structures of the bioactive compounds present in the leaves of Pterocarpus soyauxii.