Leachate Treatment by Heterogeneous Fenton on an Activated Carbon Substrate with Fe(II) Impregnated ()
1. Introduction
Generation of solid waste is due to several factors, such as population growth, industrialization, changes in lifestyle, among others. The management of this waste, mainly its final disposal, is a complex work that has become a common problem in developing countries, where it represents not only an environmental but also a social problem [1] . For the final disposal of solid waste, landfill is an economic and viable alternative in many countries. However, mismanagement of solid wastes and inadequate construction of one landfill produces pollution due to leachates generated there [2] . Leachates are rich in various pollutants, including dissolved organic matter such as volatile fatty, acids humic, acids fulvic, potentially toxic organic compounds and of difficult degradation such as xenobiotics compounds; also inorganic compounds as chlorides, nitrogen derivatives, phosphates and, metals such as Ca, Mg, Fe, Mn, Cd, Cr, Cu, Ni, Pb and Zn, among others. The volume and composition of the leachate depends on the biogeochemistry of the site, the types of waste deposited and the age of the landfill [3] . One contamination is represented by leachates in water, soil and of course to human health. The process treatment selection by these fluids is a complex task [4] [5] . Therefore, there is no exclusive treatment system for leachates; on the contrary, numerous treatment methods are usually proposed which combine and include biological processes, chemical precipitation, adsorption with granular activated carbon (GAC) and bioadsorbentes [6] , sedimentation, flotation and filtration as general treatments, as well as other specific ones such as chemical oxidation or reduction, Ion exchange, membranes, stripping and wet oxidation [7] . However, these procedures are usually inadequate to achieve the degree of purity required by local legislation. For this reason, use of the advanced oxidation (POA) is currently expanding, this consist of physicochemical process capable of producing profound changes in the chemical structure of pollutants that involve the generation and use of transient species, mainly of radical OH•, [8] and [9] .
Among the POA’s, Fenton process has been widely used for oxidation of many organic compounds due to their high efficiency to produce hydroxyl radicals from the decomposition of H2O2 in acidic medium through Fe salts. This is an attractive oxidation system because the iron is an abundant and non-toxic element, and H2O2 is easy to handle and environmentally safe [10] . Fenton oxidation can be effectively used for leachates treatment; however, this homogeneous process has certain drawbacks, such as need to recover iron salts used before final discharge [11] . As for their reaction, Fe3+ ions accumulate in the system as Fe2+ ions are consumed and reaction is finally stopped. In spite of these disadvantages presented by homogeneous Fenton process, they can be solved using solid matrices, which can be zeolites, mesoporous sieves, GAC, among others, impregnated with iron salts, which avoids the formation of sludge during the oxidation. This process is known as Heterogeneous Fenton [12] . Because of their particular characteristics, activated carbons have been widely used in heterogeneous catalysis. They can be used either as catalysts, being more important as catalyst carriers, since they fulfill most of the desirable properties (inertia, stability, adequate porosity, surface chemistry and mechanical properties) required for a suitable support. On the other hand, activated charcoals use as catalytic support presents unique advantages, such as the possibility of adapting porous structure and chemical characteristics to requirements of a specific catalyst [13] . Activated charcoal as a heterogeneous catalytic has great advantages over other solid matrices, since they are materials that have a complex porous structure, due to its large surface areas [14] . It is important to note that its surface chemistry is determined by atoms other than carbon located on edges of the heteroatom layers; where the most common are: oxygen, hydrogen and nitrogen [15] . The following are suggested reaction mechanisms in the GAC/H2O2/Fe process:

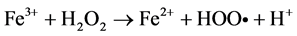
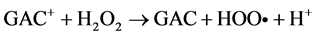
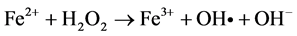

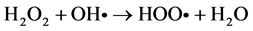
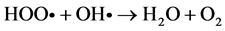
In many Fenton systems, the rate-limiting constituent in formation of hydroxyl radicals (OH•) is the ferrous ions production. Ferrous ions formation of in GAC/H2O2/Fe system can be explained by GAC functions as an electron transfer catalyst (GAC and GAC+), for which it behaves like to a donor and capable of to reduce ferric ions to ferrous ions [16] . By origin, the lignite activated carbon, contain a large amount of metals in its structure; numerous characterization studies reveal an important concentration of these in ashes [17] , [18] and [19] . This feature is very useful for the use of iron, both that which is in the structure as the impregnated on GAC surface; and them can to react with H2O2 and provide OH• radicals, to carry out the oxidation [20] . In this work a Fenton Heterogeneous advanced oxidation process was applied using GAC as a solid matrix without impregnation and impregnated with Fe2+ for the treatment of a leachate (Le), obtained from a landfill in the Merida City, Yucatán in Mexico, see Figure 1.
The objective was to determine the efficiency of Heterogeneous Fenton Process in the removal of COD and Color of crude leachates using lignitic GAC treated with acid and impregnated with Fe2+.
![]()
Figure 1. Landfill location in Mérida Yucatán, Mexico.
2. Materials and Methods
This research was carried out using leachate generated from a sanitary landfill of the Merida City, Yucatan; that began its operation 14 years ago. Samples were collected from leachates between September and December 2015 and were collected from evaporation ponds, where leachates of different ages are mixed, situation that makes treatment complex. For the sampling were used van Dorn samplers. A Granular Activated Carbon brand Carbotecnia Gamma L was used by being a lignitic granular carbon, which will be represented with acronym GAC, predominating a particle size of 9.7 ± 0.4135 mm, with 80% of mesopores and 20% macroporous, and this was characterized by chemical and spectroscopic analysis.
2.1. The GAC Pre-Treatment with HCl
Activated carbon was pretreated with concentrated hydrochloric acid (Ultrex Baker), in a ratio of 100 g of carbon per 300 ml of HCl, for a period of 8h with stirring, at room temperature, in Orbital-Shaker equipment at 100rpm, decanted and washed with hot distilled water until a constant pH of 4 ± 0.51. Subsequently it was subjected for 24 h to 105˚C; after this stage, a heat treatment to GAC was carried out at 350˚C in a muffle, in order to improve the porosity of coal [21] .
2.2. The GAC Pre-Treatment with HNO3
Treatment with HNO3 (Ultrex Baker) for GAC was important [22] , as it was intended to insert oxygen to structural surface of the coals, following the ideas of Nguyen [23] and Shi [24] , who studied that oxygen complexes can be formed on surface of activated carbon by modifying its surface chemistry. GAC’s were contacted with concentrated HNO3 in a proportion of 100 g of carbon and 300 ml of acid; it was stirred for 8 h as in HCl case. It was then decanted and resulting carbons washed successively with hot distilled water until a constant pH of 4 ± 0.42 was obtained. Activated charcoals were then dried for 24 h in an oven at 105˚C. Finally, a thermal treatment was performed to GAC’s as the case of treatment with HCl [21] .
2.3. The GAC Pre-Treatment with HCl + HNO3
A procedure was followed as in the previous two cases using 30 mL of HNO3 and 90 mL of HCl, with the same pH conditions, drying and subsequent heat treatment
2.4. Characterization of Crude Leachate
Leachate (Le) from a sanitary landfill in the city of Mérida was characterized, determining the following parameters, pH, temperature and conductivity, measured in situ with a Lab Quest field interface (Vernier, USA), according to Mexican standard: NMX-AA-008-SCFI-2011, NMX-AA-007-SCFI-2000 and NMX-AA-093-SCFI-2000, respectively. For determination of total and soluble COD, the samples were oxidized in a HI839800 reactor (HANNA Instruments, USA), according to Standard Methods 5520 C; Then a DR/2400 spectrophotometer (HACH, USA) was used, based on the Standard Methods 5520 D [25] .
BOD was determined in a FOC225E Sensor System 10 (VELP, Italy) incubator, based on Standard Methods 5210. In addition, dissolved oxygen (DO), turbidity, color, alkalinity, ammonia nitrogen (N-NH3) phosphorus, total solids (ST), total volatile solids (STV), total suspended solids (SST), volatile suspended solids (SSV). Metals such as Ca, K, Fe and Cu were determined by atomic absorption spectrophotometry in a flame atomic absorption spectrometer (AA) SOLAAR M6 (Thermo Elemental, England), after microwave digestion in a model 5 (MARS, USA), according to NMX-AA-051-SCFI-200 standard.
2.5. Characterization of Activated Carbon
2.5.1. Structural Analysis
The characterization of mesoporous GAC of lignitic origin was carried out by means of equipment ASAP 2020 Automatic Hydrocarbon-Resistant Micropore and Chemisorption Analyzer brand Micromeritics, to obtain the data of its structure, area and volume of pore. As far as characterization by scanning microscopy (SEM), it was performed in a JSM-6610 LV (JEOL, Ltd., USA) microscope. For this analysis N2 was used.
2.5.2. Chemical Analysis
Manganese, nickel, zinc and iron metals present in the GAC were determined by atomic absorption spectrophotometry by flame method in (AA) SOLAAR M6 (Thermo Elemental) equipment, with a previous acidic digestion by microwave in equipment 5 (MARS, USA), also based on the NMX standard -AA-051-SCFI- 200. 1 g of GAC both HCl-treated and HNO3 were used independently and combined. Digestion process of samples was 20 min at 175˚C.
2.6. Activated Carbon (GAC) Impregnation and Stabilized Treatment
GAC’s previously treated with HCl, HNO3 and with combined acids were impregnated with ferrous chloride (Fe Cl2∙4H2O) and ferrous sulfate (FeSO4∙7H2O), both of Aldrich brand in a ratio of 100g of carbon per 25 ± 0.35 g of FeCl2∙4H2O and 35 ± 0.37 g of FeSO4∙7H2O respectively, these values were calculated stoichiometrically. It was stabilized by lyophilization in Virtis Benchtop equipment.
2.7. Procedure for Fenton Heterogeneous and Catalytic Experiments
2.7.1. Contact of the GAC with Leachate (Le)
Experiments were carried out by Batch, taking place in 250 ml containers, suitable for 100 ml of Le and 5 g of impregnated GAC. As a control, GAC impregnated but without the pre-treatment of HCl was used, it was only washed with hot water and the procedure was followed. Leachate (Le) was adjusted to a value pH of 3 for its contact with different GAC’s for 2 h with stirring in an orbital shaker, brand Lab-Line Instruments, with controlled temperature at 150˚C and 200 rpm. Finally they were filtered in Whatman grade GF/B filters of 1 μm pore; five tests were made for each case.
2.7.2. Contact of Each GAC with Leachate (Le)
Tests were carried out at room temperature using 100 mL of Le with 5 g of each GAC treated with different acids, mentioned in GAC treatment paragraph, with continuous agitation at 200 rpm during 4 h. The pH of Le was adjusted to 3 and 5 g of pre-treated activated carbon was added.
2.7.3. Optimization Addition of Hydrogen Peroxide (H2O2)
Doses with 3.2, 6.4 and 9.6 mg/L of 30% H2O2 (Merck) were tested to remove COD and color in Le, efficiencies of these removals also were determined.
2.8. Statistical Analysis
With removal percentages of COD and color obtained in all experiments, and using statistical software package STATGRAPHICS CENTURION XVII and Fisher’s LSD method [25] , with two-way analysis of variance was performed to determine the significance of each factor, as well as an analysis of significant difference minimum (LSD).
3. Results
3.1. Characterization of the Leachate (Le)
In Table 1 presents the results of crude leachate (Le) characterization, this was obtained from Sanitary Landfill in the Merida City., this was obtained from Sanitary Landfill in the Merida City.
3.2. Impregnation of Iron (Fe2+) in Different GAC’s
Iron (II) content in impregnated GAC’s, is presented in Figure 2 and Figure 3, the GAC’s treated with HCl and washing with hot water, without impregnation, and was used as reference. Using FeSO4∙7H2O for impregnation is presented in Figure 2 and with FeCl2∙4H2O is presented in Figure 3.
Impregnations with different acids were compared in both figures, a better
![]()
Table 1. Physicochemical analysis results of Le.
![]()
Figure 2. Concentration of Fe2+ as a result of impregnation with FeSO4.7H2O in different GAC’s.
impregnation of GAC’s were obtained considering five experiments in each case, when using only HCl with respect to HNO3 and acids mixtures.
Lowest concentrations of Fe2+ were the GAC’s treated with HNO3 and acid mixture. The fact that impregnation quality decreased with HNO3 and mixtures may be due to that HNO3 somehow affects carbon surface by a possible oxidation of Fe2+ to Fe3+ from iron present in each GAC. Comparing the graphs of Figure 2 and Figure 3, it was observed that FeSO4∙7H2O gave a better impregna-
![]()
Figure 3. Concentration of Fe2+ as a result of impregnation with FeCl2.4H2O in different GAC’s.
tion of iron; however, there was not a very significant difference, as can be seen in Figure 3. For this reason FeSO4∙7H2O was selected for studies of leachates treatment by Heterogeneous Fenton method.
It is probably that Fe(OH)3 is present in aqueous medium to beginning and becoming Fe2O3 after heat treatment and this oxide could be blocking active sites for Fe2+ of the salts during impregnation. In acid mixture, considering HNO3 case, it is this acid in mixture that must be affecting the impregnation. To understand Fe(OH)3 formations, a pE-pH diagram of iron in aqueous medium is presented in Figure 4, in region of experimental conditions, where concentrated HNO3 was used and also when a pH was reached of 4.
![]()
Figure 4. pE-pH diagram of iron in water.
3.3. Dosing of H2O2 for Heterogeneous Fenton
In Table 2 and Table 3 are present values obtained for the optimization of H2O2 dosage related with the different acid treatments for activated carbons (GAC’s), in order to select the optimum H2O2 dose for leachate treatment by Heterogeneous Fenton. Parameters used for H2O2 doses were COD and the color respectively. It is observed that for COD, the best dose was 6.4 mg/L, mainly for GAC’s treatment with HCl. As for the color, with 9.6 mg/L dose, a good result was obtained, as shown in Table 3, however because 6.4 mg/L dose was the best for COD, this dose was decided for the study of both parameters in order to save H2O2..
3.4. Results of Contact with Leachate and Percentage of COD Removed
Figure 5 shows the percentage of COD removed from crude leachate (Le), where 100 mL of Le was contacted for one hour with the different GAC’s, using both
![]()
Table 2. Percentage of chemical oxygen demand (COD) removal in function of acid treatment type and peroxide dose.
![]()
Table 3. Percentage of color removal in function of acid treatment type and peroxide dose.
FeCl2∙4H2O and FeSO4∙7H2O. As in COD removal case, the Color removal was carried out using same amounts of Le, equal contact time and the same dose of H2O2, Figure 6. The two salts worked well; however FeSO4∙7H2O was selected
![]()
Figure 5. Percent removal of Chemical Oxygen Demand (COD) from leachates using GAC’s treated.
![]()
Figure 6. Percent removal of Chemical Oxygen Demand (COD) from leachates using GAC’s treated.
because it is more stable to humidity, since the State of Yucatan has a high atmospheric humidity.
3.6. Results of Surface Analysis
3.6.1. Granular Activated Carbon Surface Analysis
Brunauer-Emmett-Teller (BET) and Langmuir isotherms to determine surface areas for GAC’s, non-impregnated, acids-treated and FeSO4∙7H2O-impregnated; the isotherms indicated. Table 4 shows results of the surface analysis of GAC’s non-treatment and treatment with different acids. It is important to note that GAC treated with HCl gave the best measurements, followed by the combination of acids. Treatment with lower values was with HNO3, in this treatment a reddish layer was observed, which confirmed that Fe contained in GAC is like Fe2O3. It is important to highlight that the chief role of HCl was cleaning the GAC and eliminating metals, which resulted in more available active sites on carbon’s surface.
In Table 5, is presented results of FeSO4∙7H2O impregnated carbons, which was ferrous salt selected for impregnation, for the reasons previously indicated. The impregnated coals were treated by lyophilization to avoid disturbing the surfaces.
![]()
Table 4. Surface analysis results of non-treated GAC and GAC treated with acids.
*Washed with hot water. ***Barrett-Joyner-Halenda (BJH) Analysis.
The GAC’s treated with HCl, gave the best surface area by BET and Langmuir, the higher pore volume and sizes, what was reflected in the higher impregnation capacity, as shown in Table 5. It was possible to obtain a surface area of impregnation with Fe2SO4×7H2O of 31.05% by BET and 30.24% by Langmuir. As indicated in the porosimetry analysis, section of methods of analysis, these GAC’s were rich in mesopores, considering in the analysis (Table 4), an average of 4.26 nm, since for the mesoporous materials it is considered between 2 and 50 nm. Although also could exist macroporous size in granular lignite carbon. So it can be considered a good support for the Heterogeneous Fenton
3.6.2. Structural Analysis
Figure 7 shows images of GAC obtained by electronic scanning microscopy. Figure 7(a) shows non-impregnated GAC, and Figure 7(b) shows impregnated GAC. Red circle showcases the impregnated surface, where small granules of deposited Fe2+ can be appreciated; this image corresponds to GAC treated with:
![]()
Table 5. Surface analysis results of impregnated GAC with Fe2SO4.7H2O.
*Barrett-Joyner-Halenda (BJH) Analysis.
![]() (a) (b)
(a) (b)
Figure 7. Surface analysis of GAC treated with HCl and 6.4 mg/L doses of H2O2. (a) Non-impregnated GAC; (b) It is indicated with the red circle the small granules corresponding to the CAC impregnated with FeSO4.7H2O.
HCl, hot water-washed, and FeSO4∙7H2O impregnated.
3.7. Statistical Analysis of Variables
By means of a multiple comparison procedure, means values were determined
![]()
Table 6. Multiple range tests for percentages of COD and Color removal based on H2O2 doses.
*Fisher LSD (Least Significant Difference) method, 95% confidence interval.
![]()
Table 7. Show nine cases; the homogeneous groups indicated that the HCl treatment resulted in the highest COD and Color removal percentages, followed by treatment with HNO3.
*Fisher LSD (Least Significant Difference) method, 95% confidence interval.
which are significantly different from each other. Homogeneous groups are identified by the alignment of the sign X in each column. Within each column, the levels that have sign X form a group of means values between which there are no statistically significant differences. The method used to discern between means values was the procedure of least significant differences of Fisher (LSD).
One X was assigned in each column for differentiate the homogeneous groups. Percentages of removal of COD and Color (these are independent variables) were related to the doses of H2O2. In the same way, the percentage of removal of these independent variables was analyzed, but considering as variables for GAC, without treatment and treatment with different acid.
In Table 6, two homogeneous groups were identified according to the alignment of sign X in each column of this Table 6; the homogeneous groups showed that doses with 9.6 and 6.4 mg/L of H2O2 were ideal. In order to save reagent, 6.4 mg / L dose was used.
In Table 7, four homogeneous groups were identified according to the alignment of X sign in each column; the homogeneous groups showed that in the means of percentage elimination averages of acid treatments there are significant differences, indicating that the treatment with HCl acid was the best for Heterogeneous Fenton process.
These statistical analyses supported both the selection of H2O2 dose for the heterogeneous Fenton process and the choice of acid treatment.
4. Conclusions
After analyzing all the variables proposed in this paper, it is important to note that lignite carbon (GAC), due to its adsorbent properties, provided good chemical surface stability for Heterogeneous Fenton.
The acids pretreatments had a positive effect on the removal efficiency, particularly HCl, which presented the best efficiencies with respect to the other acids, since it does not present Fe2+ oxidation, as in the case of HNO3.
Regarding the salts, both FeSO4∙7H2O and FeCl2∙4H2O showed impregnation efficiencies above 80%, with similar reaction rates in the formation of OH• radi-
cals. However, FeSO4∙7H2O was selected because it showed the best stability against environmental humidity.
The pH with 4 values resulted in more efficient value during the impregnation on the surface of coal. Lyophilization for stability treatment by impregnation was fundamental to not alter the impregnated surface.
By means of statistical analysis a good certainty of the selection of H2O2 dose for treatment by Heterogeneous Fenton of leachate, also the best acid treatment for GAC was obtained.
Acknowledgements
To National Council for Sciences and Technology (CONACYT) which supports the PhD Graduates in Environmental Sciences and Engineering. To PhD. Sergio Gómez-Salazar researcher of the University Center in Sciences and Engineering of the University of Guadalajara by measurements specific areas to GAC’s. To PhD. Pedro Avila-Pérez, researcher of the National Institute of Nuclear Research by the analysis of scanning microscopy (SEM)