Crystal Structure, Thermal Behavior and Vibrational Spectra of 4,4’Diamoniumdiphenylmethan Sulfate Hydrate ()
1. Introduction
The chemistry of hybrid materials has received an important growth in last two decades; it was an impressive area of research for many investigations in different field of sciences [1] [2] [3] . In fact, “pillared layer” materials and three- dimensional frameworks are formed involving the sulfates ligands which promote the formation of alternating organic and inorganic layers [4] . Not only the morphologies or the length of sulfate groupments can give this chemistry its important feature; but also the chemical identity of the organic group can profoundly influence the whole structure of these compounds [5] . Presently, various syntheses of organically cation-templated sulfates crystals were the subject of many investigations which have been of great benefit in various fields of sciences [6] [7] [8] . It is worthy to note that, in general, the observed sulfate anions are, basically [HSO4]− or [SO4]2−. The cohesion forces in these hybrid compounds are dominated by electrostatic interactions, Vander Waals contacts, and hydrogen bonds (O−H・・・O and N−H・・・O). These hydrogen bonds play an important role in the mechanism of association of molecules that either biological or not [9] .
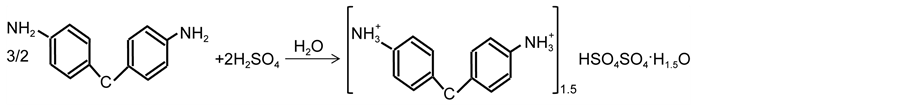
The title compound (DDPS) is an additional example for illustrating the templating effect of aromatic ammonium molecules on sulfate. In addition, structural considerations resulting from crystallographic studies, some organic sulfates exhibit non-linear optical properties [10] , or phase transitions [11] . The present work continues a series of investigations into the factors influencing the dimensions of sulfate anion-organic cation interaction. We report here the chemical preparation, crystallographic features, thermal behavior, and IR analysis of a new organic sulfate, (C13H16N2)1.5HSO4SO4・H1.5O.
2. Experimental
2.1. Synthesis
By slow evaporation single crystals of the studied compound were obtained from a solution of 4,4’-diaminodiphenylmethan [Sigma-Aldrich] and sulfuric acid (98 wt% from Fluka) as obtained from commercial sources without further purification. A solution of 4,4’-diaminodiphenylmethan (1.98 g, 0.01 mol) in 200 mL of water was added to a solution of sulfuric acid. The resulting solution was filtered through paper and left at room temperature. After several days, transparent and good single crystals were produced, which were filtered and air dried.
2.2. Characterization
2.2.1. X-Ray Single Crystal Structure Determination
The intensity data collection was performed using an Enraf-Noniusdiffracto- meter and monochromated Mo radiation (λ = 0.71073 Å). Pertinent details of the crystal structure of (C13H16N2)1.5HSO4SO4·H1.5O are listed. The strategy used for the structure determination and its final results are gathered in Table 1. The structure was solved with a direct method, from the SHELXS-97 programs, which permitted the location of the HSO4 and SO4 groups. The remaining non- hydrogen atoms were located by the successive difference.
![]()
Table 1. Crystal structure data for (C13H16N2)1.5HSO4SO4·H1.5O.
Fourier maps using the SHELXL-97 programs [12] . In the final least-squares refinement of atomic parameters with isotropic thermal factors of the hydrogen atoms, R decreased to 4.72% (Rw = 12. 60%) for DDPS. Full crystallographic information related the crystal structure has been deposited at the CCDC as CIF file, No. CCDC 1540491. Copies of this information may be obtained free of charge from the director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK (fax: +44 1223 033; deposit@ccdc.cam.ac.uk or www.http://wwwccdc.cam.ac.uk).
2.2.2. Infrared Spectroscopy
IR spectrum of DDPS was recorded at room temperature with a Biored FTS 6000 FTIR spectrometer over the wave number range of 4000 - 400 cm−1 with a resolution of about 4 cm−1. Thin, transparent pellet was made by compacting an intimate mixture obtained by shaking 2 mg of the samples in 100 mg of KBr.
2.2.3. Thermal Analysis
Setaram TG-DTA92 thermoanalyzer was used to perform thermal analysis on samples of DDPS. The TG-DTA thermograms were obtained with 21.5 mg. Samples were placed in an open platinum crucible and heated in air with 5˚C/ min heating rate; an empty crucible was used as reference.
3. Results and Discussion
3.1. DDPS Structure Description
The crystal of the title compound is built up from 4,4’-diamoniumdiphenyl- methan cations, two types of sulfate anions with different ionization state, i.e. [HSO4]− monoanions and [SO4]2− dianions, and from water molecules. Figure 1 showed that the asymmetric unit of (C13H16N2)1.5HSO4SO4·H1.5O was formed by two crystallographically different cations, two independent anions and one water molecule. The main geometrical features of different entities are indicated in Table 2.
A view of the structure projected along the b axis is reported in Figure 2. The atomic arrangement of the structure of the DDPS was described by a three-
![]()
Figure 1. Ortep drawing of the asymmetric unit of DDPS.
![]()
![]()
Table 2. Main interatomic distances (Å) and bond angles (˚) for (C13H16N2)1.5HSO4 SO4·H1.5O.
![]()
Figure 2. Projection along the b axis of the atomic arrangement in (C13H16N2)1.5 HSO4SO4·H1.5O.
dimensional network of structural units formed by clusters [HS2O8]3− sulfate, water molecules and organic cations. The mineral skeleton of this compound is formed by basic [SO4]2− and acidic [HSO4]− groups which are interconnected via O(4)−H・・・O(8) hydrogen bond leading to the formation of isolated clusters in the (a, b) plane. The same type of hydrogen bonds O−H・・・O links these mineral entities together, in pairs, via two water molecules which share a hydrogen atom located on a C2 axis, to form a finite chains parallel to c axis (Figure 3).
The short distance O(4)・・・O(8) = 2.5151(6) Å, shows that this hydrogen bond was considered strong. Distances and bond angles describing the anions [SO4]2− and [HSO4]− are illustrated in Table 2. The lengths of S−O bonds in are close to literature average [13] value of 1.4082(4) Å. S−O(H) bond in is longer (1.5520(4)
![]()
Figure 3. Projection along the b axis of the inorganic arrangement.
Å), which is typical value, however, one of the remaining S−O bonds (S1−O4; 1,5520(4) Å), is slightly shorter than the others S1−O2 and S1−O3; this fact can be explained by the location of a proton on oxygen O(4) of the anion , this characteristic is in line with those observed in the protonated oxoanions [14] [15] [16] . The mean value of S−O distances and angles O−S−O are: 1.459(4) Å, 109.37(20) and 1.458(4) Å, 109.46(19) respectively for the tetrahedra S(1)O4 and S(2)O4. These values are, also, in good agreement with those observed for similar anionic groups [17] [18] . The calculated average values of the distortion indices [19] , corresponding to the different angles and distances in both S(1)O4 and S(2)O4 tetrahedron [DI(OSO) = 0.0100 - 0.0316, DI(SO) = 0.0076 - 0.0291 and DI(OO) = 0.0602 - 0.0087] exhibit a pronounced distortion of the O−S−O angles compared to S−O and O・・・O distances. So, each sulfate group can be considered as a rigid regular arrangement of oxygen atoms, with a displacement of the S atom from its centroid. The interaction of the sulfuric acid with the organic molecule (C13H14N2) leads to the protonation of the two nitrogen atoms of the 4,4’ diaminodiphenylmethan and the formation of two cations (C13H16N2)2+ cristallographically independent, respectively denoted: A{C(1) C(7)} and B{C(8) C(20)}.
Main geometrical characteristics of these cations are summarized in Table 2. With regards to the organic cations, the A cation has a local symmetry, in which the C(1) atom is located on a C2 axis. Each sulfate anion is linked to its organic groups neighbors via N−H・・・O hydrogen bonds. Furthermore, the tetrahedron HS(1)O4 is involving in an only one O−H・・・O hydrogen bond with the water molecule and in four N−H・・・O hydrogen bond with three organic cations neighbors. The tetrahedron S(2)O4 is engaged in six hydrogen bonds of the same type from six organic cations. Thus, hydrogen bonding plays a significant role in linking the organic molecules with the anionic sheets made by HSO4 and SO4 moieties. This interaction contributes to the cohesion and the stability of the structure. All the D (donor)−H・・・A (acceptor) hydrogen bonds are listed in Table 3, with an upper limit of 2.458(7) Å for H・・・A distance and a lower limit of 87(12) ˚ for the D−H・・・A bond angles. Consequently, this atomic arrangement exhibits two types of intermolecular interactions: O−H・・・O and N−H・・・O, considered respectively as strong and weak hydrogen bonds. In this case, the titled compound contains two hydrogen bonds of the first type and ten of the second one. The N(1)−H(1)N(1)・・・O(7) interaction is considered as a strong hydrogen bond [N(1)−H(1)N(1)・・・O(7) = 1.757(6) Å] [20] . This latter provides the connection of the organic cations to the cluster [H2S2O8]2−. All these interactions play a fundamental role in the process of the creation and the stability of a three-dimen- sional framework of (C13H16N2)1.5HSO4SO4·H1.5O.
![]()
Table 3. Bond lengths (Å) and angles (˚) in the Hydrogen-bonding scheme1 of (C13H16N2)1.5 HSO4SO4·H1.5O.
1Symmetrycode: i: x − 1/2, −y + 3/2, z − 1/2; ii: −x + 3/2, y + 1/2, −z + 3/2; iii: x − 1/2, y + 3/2; ziv: −x + 3/2, −y + 3/2, −z + 2; v: x − 1/2, y + 1/2, z vi: x, −y + 1, z − 1/2; vii: x − 1, y, z.
3.2. IR Absorption Spectroscopy
Recently, several researches on the vibrational properties of organic sulfates containing HSO4 and SO4 groups had been widely investigated [21] [22] .
These studies have been focused, essentially, on the relationship between symmetry considerations and normalmodes vibrations of these groups. The common result is that these groups loose theirs free state symmetry (Td for SO4 and C3v for HSO4) to be C1 under the effect of its incorporation in the complex and its interaction with its crystalline environment. As a result, the vibrational properties are affected by moving the degeneracy of some normal modes, splitting of some IR active modes and appearance of some IR inactive modes. Here, we recall that the free ion SO4, in its ideal Td symmetry are ν1 = 981 cm−1, ν2 = 451 cm−1, ν3 = 1104 cm−1 and ν4 = 614 cm−1 [23] . In this work, an attempt to assign frequencies to different stretching vibrations and deformation of the organic cation is performed based on previous work [24] [25] . A broad band extending from 3700 to 2500 cm−1 is observed in the IR spectrum (Figure 4). This broad band must be due to the symmetric and asymmetric stretching modes of CH, NH3, NH2, OH, H2O and NH3 bending and rocking may occur in the ranges 1640 - 1520 and 933 - 722 cm−1. The out-of-plane OH bending mode ʋ (OH) appears in infrared spectrum at the average 3744 to 3780 cm−1. The shifting of the stretching and bending vibrations of the NH3 group from the free state value confirms the formation of hydrogen bonds of varying strengths in the crystal. Frequencies in the range 933 - 722 cm−1 are attributed to ρ(CH), ρ(NH3), ρ(NH2), γ(CH) and γ(C=C). The frequency bands in the region 410 - 484 cm−1 are attributed to the symmetric deformation vibration of δs(SO4). The asymmetric deformation symmetry δas(SO4) was observed in the area 602 - 671 cm−1.
![]()
Figure 4. IR Spectrum of (C13H16N2)1.5HSO4SO4·H1.5O.
While that connected to the symmetry of valence SO4 group is presented by the band 992 cm−1. Bands observed in the 992 - 1186 cm−1, on asymmetrical valence vibration νs(SO4) δas(SO4) region.
3.3. Thermal Analysis
The simultaneous TG?DTA analysis curves of (C13H16N2)1.5HSO4SO4·H1.5O were carried out in air at a heating rate of 5˚C/min on a sample of 21.5 mg placed in a platinum crucible and heated from ambient to 325˚C. The result obtained during the decomposition of the title compound is illustrated in Figure 5. This study shows that this complex is thermally stable up to 185˚C. The TGA curve revealed the presence of two steps weight losses. The first decomposition, from 185˚C to 205˚C which is accompanied by one shoulder on the DTA curve at 197˚C, corresponds to the loss of the water molecules (experimental weight loss: 3.72% and theoretical weight loss: 3.42%) and leading to anhydrous framework of (C13H16N2)1.5HSO4SO4. The second stage, starts at about 220˚C and ends above 300˚C, is assigned to the degradation of the organic entities (experimental loss: 65.46% and theoretical loss: 60.85%). This phenomenon is accompanied by one endothermic peak observed on the DTA curve at about 238˚C.
4. Conclusions
The crystals of the 4,4’-diamoniumdiphenylmethan sulfate hydrate, (C13H16N2)1.5 HSO4SO4·H1.5O, have been synthesized by slow evaporation method at room temperature. The X-ray diffraction helped us to determine the cell parameters and the space group. The structural study showed that the anionic groups SO4 and HSO4 are gathered by inter-anionic strong hydrogen bonds giving birth to clusters
![]()
Figure 5. TG-DTA thermograms of (C13H16N2)1.5HSO4SO4·H1.5O.
[HS2O8]3−. It is also shown the scheme of hydrogen bonds connecting various organic cations with the sulfates clusters to yield a three-dimensional network.
The detailed vibrational spectral analysis, supported by an analysis using group theory, of the organic and the inorganic moieties was carried out leading to the counting and the allocation of frequencies characteristic of the compound.
The TG-DTA data allowed us to verify that the title salt is thermally stable up to 185˚C. The stability of the compound below this temperature can be explained by the different strong bonds observed by the X-ray diffraction. The thermal decomposition above 185˚C takes place in two steps. The endothermic peaks at 197˚C and 238˚C are due to the thermal decomposition with release of the uncoordinated water and degradation of organic entities.
Moreover, a study of the electrical conductivity of the reported compound could open to it opportunities on applied plan.
Appendix A. Supplementary Data
The final atomic coordinate and thermal parameters are given in Table S1. Those of hydrogen-atoms were also determined but are not given, in order to shorten the table.
![]()
Table S1. The final atomic coordinates and equivalent temperature factors for (C13H16N2)1.5HSO4SO4·H1.5O.