1. Introduction
Greenhouse effect is recognized as one serious threat to the environment. It would increase the global average temperature and make the sea level raised [1] . As the results, the land supporting billions of people would be submerged. CO2 is a main factor for the heightening greenhouse effect, because it contributed about 64% to the total radiative forcings from the long-lived and well mixed greenhouse gases (CO2, CH4, N2O and halogenated compounds) in 2011 [2] . Over 32.1 Gt CO2 was emitted from fossil fuel combustion in 2013 [3] . To lessen fossil fuel consumption is a direct and effective method to decrease CO2 emission, however, it can only be realized when sustainable fuels are supplied to replace the fossil fuels. Methanol, dimethyl ether and hydrocarbons are the products of CO2 hydrogenation [4] - [12] which are able to be used directly as fuels. In case H2 is generated from renewable energy source such as biomass or solar energy [4] [5] [13] , the process of CO2 hydrogenation is CO2-neutral besides to supply sustainable fuels. Such process will solve fuel lack of isolate islands by recovering CO2 from seawater [14] .
On iron catalyst, CO2 can be hydrogenated into hydrocarbons by two steps as shown in the following Equation (1) and (2) [9] [10] [11] . CO2 is converted into CO by reverse water-gas-shift (WGS) reaction, and then the produced CO is further hydrogenated to hydrocarbons by Fischer-Tropsch (FT) reaction.
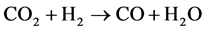 (1)
(1)
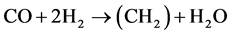 (2)
(2)
WGS reaction is thermodynamically favored at low temperature [15] . Therefore, reverse WGS reaction is favored at high temperature, i.e. high temperature can promote CO2 conversion to hydrocarbons via the series of Equations ((1) and (2)). In order to find iron catalyst more active for CO2 hydrogenation, we researched the influences of Zn, K and Cu on precipitated iron catalysts at low temperature (230˚C) [12] [16] [17] . The functions of Zn and K to activate CO2 into CO were explained based on the experimental data from CO2 temperature-programmed desorption [16] . Recently, the catalysts were evaluated at increased reaction temperature, and the catalyst filling constitutions in the reactor were compared in order to increase hydrocarbon yield as high as possible.
2. Experimental
2.1. Catalyst Preparation
All the used reagents are analytical grade (Sinopharm Chemical Reagent Co., Ltd.). Catalysts were prepared in two methods. Method I was to precipitate a mixed solution of Fe(NO3)3 and potassium silicate with (NH4)2CO3 solution at 50˚C and pH = 6.5 ± 0.5. Method II was to precipitate Fe(NO3)3 solution with the mixed solution of (NH4)2 CO3 and potassium silicate at 50˚C and pH = 6.5 ± 0.5. Then, the precipitate was washed with distilled water and centrifuged at 4500 RPM for three times after 1 h ageing at room temperature. Promoters of Zn, K and Cu were impregnated onto the precipitate with Zn(NO3)2, KNO3, and Cu(NO3)2 solution. After the catalyst precursor was spray-dried at 250˚C and calcined at 360˚C for 6 h, it was shaped into particle of 80 - 150 μm for activity test. The obtained catalysts are expressed as ZlKmCn/FSr-I or ZlKmCn/FSr-II according to the method to introduce SiO2. In the above abbreviation of catalysts, Z, K, C, F, and S represent Zn, K, Cu, Fe, and SiO2, respectively. L, m, n, and r are the nominal mass percent of corresponding materials relative to Fe2O3.
2.2. Characterizations
BET surface area, pore volume and average porediameter of the catalysts were measured using ASAP-2020 from Micromeritics at −196˚C. The crystal structure of catalysts was analyzed by PNAlytical diffractometer (X’Pert Pro) with a Cu Kα radiation source (λ = 0.15406 nm).
2.3. Activity Test and Product Analysis
The activity of catalysts was tested in a stainless steel fixed bed reactor [12] . The catalyst diluted with four-fold quartz particle of equal size was filled into the reactor. After the catalysts were reduced in CO of 50 mL∙min−1 at 300˚C for 6 h, it was cooled to room temperature. Then, the feed gas was changed into reactants (64% H2/32%CO2/4%N2) of 1.6 MPa. The catalyst was heated to reaction temperature in about 3 h and kept at it for 45 h. The detail parameters for activity test are given with the experimental results.
C5+ hydrocarbons were collected in an ice trap of 0˚C at system pressure. The distribution of C5+ hydrocarbons was measured off line by GC-9860 (Qiyang Ltd.) with FID detector and OV-1701 capillary column. After the system pressure was released through a backpressure regulator, the exited gas was analyzed on line. The quantities of CO, CH4, CO2 and N2 were supplied with TCD detector and TDX-01 column. N2 was used as an internal standard for the quantitation with the GC/TCD. C1 - C5 hydrocarbons were analyzed with FID detector and Al2O3 capillary column [18] .
3. Results and Discussion
3.1. Influence of SiO2 Content in Catalyst
Different to the iron catalysts studied in our previous works [19] [20] [21] , SiO2 is used as structure promoter in this work. Figure 1 contrasts the influence of SiO2 content on the catalysts’ activity for both of CO2 hydrogenation (CO2 conversion) and CO hydrogenation (CO conversion). These catalysts were prepared in Method I, and the content of Zn, K and Cu is 8 wt%, 5 wt% and 4 wt%, respectively. The catalyst with 15 wt% SiO2 is the most active one for CO2 hydrogenation, while this catalyst shows the lowest CO conversion in CO hydrogenation.
Table 1 gives the data of catalysts’ texture. With raised SiO2 content in the catalysts,
![]() For CO2 hydrogenation: H2/CO2 =2, 230˚C, 1.6 MPa, 6.0 L・h−1∙g-cat.−1. For CO hydrogenation: H2/CO =1.5, 235˚C, 1.6 MPa, 3.0 L・h−1∙g-cat−1
For CO2 hydrogenation: H2/CO2 =2, 230˚C, 1.6 MPa, 6.0 L・h−1∙g-cat.−1. For CO hydrogenation: H2/CO =1.5, 235˚C, 1.6 MPa, 3.0 L・h−1∙g-cat−1
Figure 1.Influence of SiO2 content on catalyst activity in CO2 hydrogenation and CO hydrogenation.
![]()
Table 1. BET surface area and pore size.
aIt is calculated by BJH method from the desorption branch.
the specific surface area, pore volume and average pore diameter increase monotonically which do not match to either the volcano shape of CO2 conversion or reverse volcano shape of CO conversion in Figure 1. The enlarged surface area usually reflects increased dispersive degree of every component in the catalysts.
Figure 2 is the XRD results of the four catalysts. There are peaks around 23.92˚, 33.48˚, 35.88˚, 40.72˚, 49.68˚, 54.08˚, 62.76˚ and 63.76˚ in Z8K5C4/FS5-I, Z8K5C4/ FS10-I and Z8K5C4/FS20-I. They are assigned to Fe2O3 (JCPDS 89-0597). These peaks almost disappear in Z8K5C4/FS15-I. The disappearance of crystalline Fe2O3 indicates that iron is highly dispersed by the added SiO2 in Z8K5C4/FS15-I. This dispersing effect of SiO2 is happened to the promoters, too. It has been reported that the amount of effective potassium is decreased by the addition of SiO2 into iron catalysts because of K-Si interaction [22] [23] . Therefore, the amount of effective potassium is probably the lowest in Z8K5C4/FS15-I. It results in effective Zn/K ratio higher than the nominal Zn/K ratio calculated by the added amounts of zinc and potassium. We have disclosed that the iron catalyst with higher Zn/K ratio is more active to hydrogenate CO2 [12] [16] .
Table 2 lists the ethene and propene ratio in the C2 and C3 hydrocarbons (sum of olefin and paraffin), respectively. For CO hydrogenation, both of the ethene and propene content are almost constant in spite of the SiO2 content is different for the studied catalysts. It indicates that the ratio of adsorbed carbon atom (Cad) to adsorbed hydrogen atom (Had) [24] is independent of the SiO2 content in catalysts. Contrarily, for CO2 hydrogenation, the ethene and propene content decrease with SiO2 content raised in the studied catalysts. It reflects that the ratio of Cad to Had is declined by the added SiO2 for CO2 hydrogenation. According to the two steps to hydrogenate CO2 into hydrocarbons
![]() A: Z8K5C4/FS5-I, B: Z8K5C4/FS10-I, C: Z8K5C4/FS15-I, D: Z8K5C4/FS20-I
A: Z8K5C4/FS5-I, B: Z8K5C4/FS10-I, C: Z8K5C4/FS15-I, D: Z8K5C4/FS20-I
Figure 2. XRD pattern of the catalysts with different SiO2 content.
![]()
Table 2. Olefin ratio in C2 and C3 hydrocarbons.
The reaction conditions are as same as those shown in the caption of Figure 1.
[9] [10] [11] , the Cad is from the CO which was evolved from CO2, rather than directly from the adsorbed CO2. On the same iron catalyst, the Had quantity in CO2 hydrogenation is probably as same as in CO hydrogenation. Therefore, it can be thought that the co-adsorption of CO2 and CO on iron catalyst induces decreased Cad. However, it is only an assumption at present. We are trying to measure the Cad and Had quantity after CO and H2 or CO2 and H2 are co-fed to the iron catalysts.
3.2. Influence of Reaction Temperature
Catalyst Z2K3C4/FS5-I was evaluated under different reaction temperature. The results are given in Table 3. CO2 conversion is increased continually with raised reaction temperature. With reaction temperature increased, the equilibrium of Equation (1) shifts to the right which is beneficial to supply CO for FT reaction according to Equation (2). It had been found that the extent of CO converted to hydrocarbons increased with raised reaction temperature [22] [25] . Both of CO selectivity and CH4 selectivity show an evident decrease from 230˚C to 240˚C, but there is no monodirectional change under higher temperature. The ethene content in C2 hydrocarbons increases with raised reaction temperature. Under high temperature, the residence time of adsorbed ethene on the catalysts would be shortened due to the enhanced thermal motion. It leads to decreased probability for the adsorbed ethene to initiate carbon-chain growth or be hydrogenated into ethane.
The observed phenomenon about ethene selectivity in Table 3 is different from the results found in CO hydrogenation, where the ethene selectivity was decreased with increased reaction temperature [25] [26] . Combining the above difference between CO2 hydrogenation (Table 3) and CO hydrogenation [25] [26] with the disparity shown in Table 2, much research is needed to understand the reactive mechanism of CO2 hydrogenation on iron catalyst.
3.3. Comparison of Catalyst Filling Constitution in the Reactor
For CO2 hydrogenation in a fixed bed reactor, the H2O and hydrocarbons produced in the upper part of catalyst bed can absorb on the downstream catalyst, which probably influence the reaction of remained CO2 and H2 on the downstream catalyst. The influences are examined using catalyst Z8K3C6/FS5-I and Z8K3C6/FS10-II. Two constitutions are adopted to fill the catalysts as described in Figure 3. Constitution I is to install
![]()
Table 3. Influence of reaction temperature on CO2 hydrogenation.
H2/CO2 = 2, 1.6 MPa, 6.0 L∙h−1∙g-cat.−1
![]()
Figure 3. Principal scheme of catalyst filling constitutions.
![]()
Table 4. CO2 hydrogenation with different catalyst filling constitution.
H2/CO2 = 2, 280˚C, 1.6 MPa, 3.0 L・h−1・g-cat.−1
Z8K3C6/FS10-II directly to the downstream of Z8K3C6/FS5-I, on the contrary, Constitution II is to insert one 0˚C trap between Z8K3C6/FS5-I and Z8K3C6/FS10-II to collect H2O and condensable hydrocarbons produced by the upper Z8K3C6/FS5-I.
Table 4 contrasts the reaction data from the two constitutions. Relative to the results of Constitution I, CO2 conversion, CH4 selectivity and C2 - C4 selectivity are increased in Constitution II. For Constitution I, the H2O and long-chain hydrocarbons produced by catalyst Z8K3C6/FS5-I may adsorb on Z8K3C6/FS10-II. Their re-adsorptions on Z8K3C6/FS10-II occupy some sites which can activate CO2. Such occupancy inhibits CO2 activation and reaction on Z8K3C6/FS10-II. The occupancy also impairs H2 adsorption on Z8K3C6/FS10-II, which is indicated by the lower CH4 selectivity and higher ethene ratio in C2 hydrocarbons for Constitution I than Constitution II. However, it is beneficial for carbon-chain growth to produce long chain hydrocarbons. As listed in Table 4, Constitution I has a higher C5+ yield than Constitution II.
Figure 4 compares the distribution of liquid hydrocarbons from the two Constitutions. There is an increase from C5 hydrocarbons to C8 hydrocarbons. It does not mean that the selectivity of C5, C6 and C7 hydrocarbons is less than C8 hydrocarbons, because some molecule of C5 - C7 hydrocarbons cannot be completely condensed in the cold trap and are emitted with the exited gas. The C5 - C7 hydrocarbons had been found by the on line GC/FID designed to analyze the gaseous hydrocarbons. The hydrocarbon selectivity of FT reaction usually decreases with elongated carbon chain [27] [28] . However, the selectivity for the hydrocarbons in C20 - C24 is higher than that of C15 - C19
![]() H2/CO2 =2, 280˚C, 1.6 MPa, 3.0 L・h−1・g-cat.−1 ▲: Constitution I, ■: Constitution II.
H2/CO2 =2, 280˚C, 1.6 MPa, 3.0 L・h−1・g-cat.−1 ▲: Constitution I, ■: Constitution II.
Figure 4. Influence of catalyst filling constitution on liquid hydrocarbons distribution.
hydrocarbons for Constitution I. This phenomenon may result from the olefin re-ad- sorption [27] which enhances the hydrocarbon growth into long carbon chain.
Considering the simplicity of reactor structure, Constitution I is an optimum type to synthesize liquid hydrocarbons from CO2 hydrogenation.
4. Conclusion
Iron catalysts were prepared by co-precipitation of Fe and Si. Zn, K and Cu were impregnated to FeSi precipitate as promoters. For CO2 hydrogenation reaction, the catalyst with 15 wt% SiO2 possesses the highest CO2 conversion. The olefin content in C2 and C3 hydrocarbons is lower in CO2 hydrogenation than in CO hydrogenation which may be influenced by the co-adsorption of CO2 and CO on the catalyst. CO2 conversion and ethene selectivity are increased with raised reaction temperature. For fixed bed reactor, the H2O and hydrocarbons produced in the upper catalyst may re-adsorb on the downstream catalyst, and it is beneficial for carbon-chain growth by inhibiting H2 adsorption.
Acknowledgements
This work is supported by Zhejiang Provincial Natural Science Foundation of China (LY14B030003), and National Ministry of Science and Technology of China (2014BAD02B05).