Atrazine Sorption by Biochar, Tire Chips, and Steel Slag as Media for Blind Inlets: A Kinetic and Isotherm Sorption Approach ()
1. Introduction
The Conservation Effects Assessment Project (CEAP) is a U.S. Department of Agriculture multi-agency effort to evaluate the environmental benefits of conservation practices and to develop new and/or modify the existing ones to manage the agricultural landscape for environmental stewardship. These conservation practices include reduced tillage, nutrient management, buffer strips, and drainage water management, among others.
Fields with subsurface drainage systems and prolonged standing water in depressional areas can drastically reduce crop yields. This concern can be partially alleviated by installing surface inlets that reduce the duration of ponding [1] . Unfortunately, these inlets provide an open conduit for surface water to enter subsurface drains, and thus can contribute to water quality concerns. Blind inlets are a relatively new conservation practice designed to replace surface inlets and reduce these concerns while maintaining high infiltration rates. They are infiltration galleries constructed by covering perforated drainage pipes with gravel followed by a geotextile membrane and topped with a layer of sand. Blind inlets have added benefits, compared to traditional tile risers (vertical perforated pipes) including reduced sediment losses and farming operations can be conducted over the blind inlets, including tillage, planting, and harvesting [2] . Moreover, blind inlets, compared to tile risers, have been shown to reduce annual losses of phosphate-P (up to 72%), ammonium-N (up to 59%), nitrate-N (up to 24%) [2] [3] ; and herbicides including atrazine (up to 82%), metolachlor (up to 80%), 2,4-D (up to 81%), and glyphosate (up to 72%) [4] .
The effectiveness of blind inlets, compared to tile risers, in reducing sediment, nutrient, and pesticide losses is attributed to the tortuosity created by the fill material (typically readily available limestone) and the accumulation of sediments, which may serve as sorption sites for nutrients and pesticides [2] [4] . The lifespan of blind inlets is unknown; however, blind inlets in small-farmed closed depressions in Northeast Indiana were installed in 2005, and they are still functioning after more than ten years [2] [4] . Even though the current designs of blind inlets are effective at reducing sediment and pollutant losses from surface runoff, replacing some of the limestone, a sedimentary rock composed mainly of CaCO3, with stronger sorbents such as scrap tires, biochar, and steel slag might enhance their performance.
About 3.47 × 106 Mg of scrap tires were generated in 2013 in the U.S., of which 2.97 × 105 Mg were disposed of in landfills [5] . Scrap tires have shown to be effective sorbents of heavy metals [6] [7] , aromatic hydrocarbon compounds [8] [9] ; and pesticides [10] . Tires are constructed using textile, steel, and rubber with the type and amount of each component dependent on the manufacturer and tire usage [11] . The rubber composites are made of synthetic and natural rubber and reinforcing materials that include carbon black [9] [12] , which may be a key component for the sorption of aromatic compounds.
Biochar is a carbonaceous material produced from feedstock pyrolysis in low oxygen environments. The chemical and physical properties of biochars are dependent on feedstock type and pyrolysis conditions. As pyrolysis temperatures increase, feedstock characteristics are diminished, and biochar resembles condensed carbonaceous matter [13] . As a soil amendment, biochar can improve chemical and physical properties of unproductive, dystrophic soils [13] [14] . Another benefit is that biochar sorbs inorganic and organic compounds of environmental concern, including heavy metals and pesticides [15] [16] [17] [18] . Biochar is currently available in small batches, but it may be available in large quantities as a byproduct of biofuel production if this industry thrives in the future.
Electric arc steel furnace slag (steel slag) is a byproduct of the smelting of iron ore to produce steel. Steel slag is composed mostly of oxides of Ca, Fe, and Al. Steel slag is currently being studied as a blind inlet bed material to remove soluble P from surface runoff.
Atrazine, a pre- and post-emergence herbicide to prevent broadleaf weeds in corn (Zea mays), is the second most used herbicide in corn; in the U.S. Midwest, 12,271 Mg were applied in 2014 [19] . Atrazine off-transport to streams is of environmental concern since concentrations can exceed both aquatic life benchmarks [20] and threshold levels in drinking water supplies [21] . Atrazine is the most frequently detected pesticide in agricultural streams [22] . Thus, readily available material that sorbs specific compounds (e.g. atrazine) may be valuable as an alternative or add-on to the limestone to enhance the blind inlet potential to reduce losses of pollutants that otherwise may end up in streams. These alternative materials should not pose environmental concerns when used in the field, e.g. leaching of other pollutants.
In this study, we investigated atrazine sorption by tire chips, steel slag, and biochar that could be used in the blind inlet as bed materials. In addition, we measured their potential to leach other contaminants using standard protocols to ensure that their usage does not result in environmental concerns.
2. Materials and Methods
2.1. Media
The tire chips (Entech, White Pigeon, MI, USA) were ~2 × 4 cm in size and included a mixture of ~50% “tire chips with fabric” (cross woven pieces of fabric exposed on edges of the tire chip), ~25% “layered tire chips” (layers of exposed fabric, less than 5 mm in length), ~20% “tire chips with metal” (metal pieces exposed), and ~5% “rubber tire chips” (no secondary material exposed). The steel slag, produced at Nucor Steel (Crawfordsville, IN, USA) and provided by Edward C. Levy Co. (Dearborn, MI, USA), ranged from 0.75 to 1.25 cm in length and the mineral composition included larnite (Ca2SiO4), srebrodolskite (Ca2Fe2O5), brownmillerite (Ca2AlFeO5), wuestite (FeO with MgO and MnO), gehlenite (Ca2Al2SiO7), and bredigite (Ca7Mg(SiO4)4) [23] . The limestone (Hanson Aggregates, Fort Wayne, IN, USA) ranged from 0.75 to 1.25 cm. The biochar (Buy Activated Charcoal, Crawford, NE, USA), was derived from oak (Quercus spp.) and pyrolyzed at 425˚C with a particle size of 0.5 to 1 cm. Each material, except biochar, was triple rinsed in deionized (D.I.) water to remove any fines and allowed to air dry (Figure 1).
2.2. Sorption Studies
2.2.1. Kinetic Sorption Studies
Kinetic studies were conducted only for the tire chips and biochar. A 10 µg∙L−1 solution of atrazine (prepared in 0.01 M CaCl2) was added to biochar (1:32 biochar/atrazine solution ratio, w/w) or tire chips (1:5 tire chips/atrazine solution ratio, v/v) and shaken at room temperature in a reciprocal shaker. The different sorbent/solution ratios were employed due to the small quantities of biochar available for the experiment (compared to the tire chips). Subsamples from the biochar experiment were withdrawn at 1, 2, 3, 4, 6, 8, 10, 12, 14, 18, 22, and 26 hours; whereas from the tire chips subsamples were withdrawn at 1, 2, 3, 4, 6, 8, 10, 12, 24, and 28 hours, filtered through 0.45 µm nylon filters and refrigerated at 4˚C prior to atrazine analysis. Three replicates were run for each material.
![]()
Figure 1. Material used in this study; (a) biochar; (b) steel slag; (c) limestone; and (d) rubber tires chips; (e) tire chips with fabric; (f) layered tire chips; and (g) tire chips with metal.
2.2.2. Sorption Isotherm Studies
About 100 cm3 of tire chips, limestone, or steel slag and 300 mL of 0.01 M CaCl2 (or D.I. water for limestone and steel slag) for were placed into 1-L HDPE containers and pre-equilibrated using a gyratory shaker at 100 rpm overnight. After pre-equilibration, pH of the solutions was measured, and the solution was decanted. Atrazine solutions, prepared in 0.01 M CaCl2 (or D.I. water for limestone and steel slag) to target a total concentration of 0, 3, 15, 30, 150, 300 and 1500 µg∙L−1 of atrazine at a 1:5 material/solution ratio (v/v), were added to the sorbing materials and shaken on a gyratory shaker at 100 rpm for 24 hours. After the sorption step, the samples were analyzed for pH, filtered through 0.45 µm nylon filters, and refrigerated at 4˚C before atrazine analysis. For the desorption step, 500 mL of 0.01 M CaCl2 was added to the samples after the sorption step and shaken at 100 rpm for 24 hours on a gyratory shaker. The supernatant was analyzed for pH, filtered through 0.45 µm nylon filters, and refrigerated at 4˚C before atrazine analysis. For the biochar, atrazine solutions prepared in 0.01 M CaCl2 and biochar (1:32 biochar/atrazine solution ratio, w/w) were shaken for 24 hours at room temperature in a reciprocal shaker to target 5, 10, 50 and 100 µg∙L−1 of atrazine, filtered through 0.45 µm nylon filters, and refrigerated at 4˚C prior to atrazine analysis. Three replicates were used for each material and concentration level.
2.2.3. Atrazine Sorption by Dry and “Pre-Wetted” Tire Chips
During long periods without rain, the blind inlet bed material becomes dry; thus, a study was conducted to determine if the “wetting” state of the tire chips affected atrazine sorption. About 100 cm3 of mixed tire chips and 400 mL of 0.01 M CaCl2 solution were placed in 1-L HDPE bottles and pre-equilibrated overnight (“pre-wetted” state) on a gyratory shaker at 100 rpm. After pre-equilibration, 100 mL of atrazine were added, to yield final concentrations of 50 and 100 µg∙L−1, and shaken on a gyratory shaker at 100 rpm for 24 hours. For the “dry” state, about 100 cm3 of dry tire chips + 500 mL of 50 or 100 µg∙L−1 atrazine were added in 1-L HDPE bottles and shaken on a gyratory shaker at 100 rpm for 24 hours. For both the “pre-wetted” and “dry” states, after the sorption step, a sub-sample was filtered through a 0.45 µm nylon filter and refrigerated at 4˚C before atrazine analysis. The remainder of the supernatant was decanted. For the desorption step, 500 mL of 0.01 M CaCl2 were added to the tire chips and shaken for 24 hours at room temperature. After 24 hours, the samples were filtered through 0.45 µm nylon filters and refrigerated at 4˚C before atrazine analysis. Three replicates were used for each concentration level.
2.3. Atrazine Analysis
Samples were analyzed for atrazine using gas or liquid chromatography. Samples from biochar and tire chip sorption experiments were analyzed using a gas chromatography system equipped with a mass spectrometer detector [24] . Samples from the steel slag, limestone, and the “pre-wetted vs. dry” experiments were analyzed using an ultra-per- formance liquid chromatography system equipped with tandem mass spectrometer detectors [4] .
2.4. Sorption Parameters
The kinetic sorption data was fitted to the pseudo first- and second-order reaction models. Pseudo kinetic reaction models are used to fit experimental kinetic data from liquid-solid interfaces [25] . The sorption rate under the pseudo first-order reaction equation is stated as [26] :
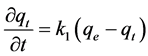 (1)
(1)
where qe is the amount sorbed at equilibrium (µg∙kg−1), qt is the amount sorbed at time t (µg∙kg−1), and k1 is the pseudo first-order reaction (min−1). Integration of Equation (1) under the conditions qt = 0 at t = 0 and qt = qt at t = t:
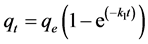 (2)
(2)
The “Exponential Rise to Maximum equation, Single, 2 Parameter” nonlinear regression 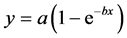 in SigmaPlot 11 (Systat Software, Inc., San Jose, CA) was used to as equivalent to Equation (2) to fit the experimental data [26] .
in SigmaPlot 11 (Systat Software, Inc., San Jose, CA) was used to as equivalent to Equation (2) to fit the experimental data [26] .
The pseudo second-order reaction model is written as:
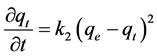 (3)
(3)
where k2 is the pseudo second-order rate constant (µg∙kg−1∙min−1). Integration of Equation (3) gives [26] :
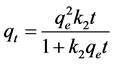 (4)
(4)
The “Hyperbola equation, Single Rectangular, 2 Parameter” nonlinear regression from SigmaPlot 11 is given by:
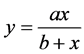 (5)
(5)
Thus, Equation (4) can be rearranged to Equation (5):
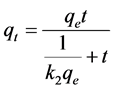 (6)
(6)
The sorption data was fitted to the Freundlich equation:
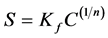 (7)
(7)
where S is the sorption (mg∙kg−1), C is the equilibrium concentration (mg∙L−1), and the dimensionless Kf is the sorption parameter, and n is a linearity parameter [27] .
2.5. Leachability Studies
To investigate the leachability of metals of environmental concern from tire chips, steel slag, and limestone used in this study, we used the US EPA 1312 procedure Synthetic Precipitation Leaching Procedure (SPLP) [28] and 1311 Toxicity Characteristic Leaching Procedure (TCLP) [29] . The SPLP test is used to simulate in-situ leaching from the testing material under synthetic precipitation, whereas the TCLP is used to simulate leaching under landfill conditions. Briefly, 25 g of sample was combined with 500 mL of SPLP (pH 4.2) or TCLP solutions and shaken on a reciprocal shaker at 90 rpm for 24 hours. A 20 mL sample was removed for pH evaluation, and the remaining solution was filtered using a 0.45 µm nylon filter and refrigerated at 4˚C before metal analysis using a Perkin-Elmer Optima 8300 inductively coupled plasma optical emission spectrometer system (Waltham, MA).
3. Results and Discussion
3.1. Kinetic Studies
The data from the kinetic sorption studies for the biochar and tire chips were well fitted to the pseudo first- and second-order reaction models (Figure 2). The correlation coefficients for each model, however, were higher for biochar than for tire chips indicating a better fit for biochar. Within materials, the correlation coefficients for the pseudo
![]()
![]()
Figure 2. Measured cumulative sorption of atrazine by biochar and tire chips as a function of time fitted to pseudo first- and second-order models. Error bars represent the results of three replications.
first- and second-order reaction models were similar (Figure 2), suggesting that either model can be used. The rate of sorption was ~38 times faster for biochar than the tire chips and equilibrium was reached in ~6 hours compared to ~30 hours (as calculated from by the first-order kinetic model) for tire chips. In the first hour, 97% of the atrazine was sorbed by the biochar and after 6 hours, 99% of atrazine was removed from the solution. In comparison, tire chips removed only 18% and 23% after 1 and 6 hours, respectively. After 24 hours, tire chips removed 50% of the atrazine from solution.
The kinetic sorption of organic compounds on porous materials is governed by fast and slow sorption steps, indicative of surface sorption and pore-diffusion processes, respectively [30] . Thus, plotting kinetic sorption data provides information on the sorption processes. With biochar, fast atrazine sorption occurred within two hours, followed by slow sorption. For the tire chips, atrazine sorption rate decreased with time as observed by the convex curve (Figure 2). Given the fact that >93% of atrazine sorption by biochar occurred during the first hour the data suggested that surface sorption was the dominant process whereas atrazine sorption by tire chips may have been controlled by both surface sorption and pore-diffusion processes.
Reports on kinetic sorption of atrazine by biochar and tire chips are scarce. Gupta and coworkers [31] using activated charcoal prepared from waste rubber tire, reported that a pseudo first-order reaction model explained atrazine sorption kinetics better than a pseudo second-order model. Their pseudo first-order rate constants (i.e. k1 values) were, 5.59 and 3.65 h−1, for initial atrazine concentrations of 5 and 12 mg∙L−1, respectively. These values are similar to the calculated k1 value in our study, 3.08 h−1 for biochar. Conversely, for atrazine sorption kinetics by granulated activated carbon and carbon nanotubes using atrazine concentrations of 1 and 30 mg∙L−1, the k1 and second- order reaction rate constant (k2) values ranged from 0.038 to 0.042 h−1 and from 8.00 × 10−9 to 1.89 × 10−7 kg∙µg∙h−1, respectively [30] . In a biochar-amended soil (1% w/w), using an initial concentration of 5 mg∙L−1 atrazine, the k2 values ranged from 3.11 × 10−5 to 7.46 × 10−5 kg∙µg∙h−1, and the amount sorbed at equilibrium was dependent on the biochar used [32] . Biochars produced at higher temperatures reached equilibrium faster than biochars produced at lower temperatures [31] . In our study, we used an initial atrazine concentration of 10 µg∙L−1 to reflect concentrations typically observed in surface runoff water. Conversely, the above-cited studies used initial atrazine concentrations ranging from 1 to 30 mg∙L−1 with a variety of sorbents (biochar, activated carbon, and carbon nanotubes). This probably contributed to the differences in the rate constant values we calculated and those reported in these studies.
Alam and coworkers [33] studied atrazine sorption kinetics for scrap tire granules (size ~0.15 to 0.30 mm) and observed that the pseudo-first order k1 values ranged from 0.72 to 0.81 h−1 and increased slightly with initial atrazine concentrations of 0.5 to 7.5 mg∙L−1. Furthermore, from 84% to 88% of the atrazine in solution (initial concentration 0.5 or 1.0 mg∙L−1, respectively) was removed with only three-hour shaking [34] . The higher k1 values obtained by these authors, compared to the tire chips in our study, 0.08 h−1, may be explained by the size of the material. In our study, tire chips measured from 2 to 4 cm in size (Figure 1); whereas Alam and coworkers used rubber granules that were 67 to 267 times smaller in size. As a general rule, surface area increases as the material size decreases; thus, more sorption sites available and the faster the kinetic sorption.
3.2. Sorption Isotherms
Sorption studies were conducted by using about 100 cm3 of tire chips, limestone, and steel slag. The particle density of these materials was different with tire chips at 1.10 g∙cm−3, limestone 2.43 g∙cm−3, steel slag 3.51 g∙cm−3 and with biochar at only 0.67 g∙cm−3. Therefore, the Freundlich sorption parameters were calculated based on the mass of the materials instead of volume. Based on the Freundlich parameters (Table 1), atrazine sorption was highly dependent on sorbent type (Figure 3). Biochar was considerably more effective in removing atrazine in solution than the tire chips, limestone, and steel slag.
Atrazine has a high affinity to sorb on biochar with the extent of the sorption dependent on several factors including feedstock type and pyrolysis conditions [18] [35] [36] . Higher Kf values for biochar were obtained in our study, log Kf = 6.79, than those in the literature, log Kf from 2.25 to 3.15, using biochars derived at 450˚C from trees, corncob, or corn straw [18] [35] [36] . The reasons for our higher sorption parameters are uncertain; however, we used lower initial concentrations of atrazine (compared to 0.05 to 30 mg∙L−1 in the cited studies), which may contribute to the faster equilibrium. The specific details of the biochar used in our experiment are unknown; however, this biochar is used for filtration (Buyactivatedcharcoal.com),which may suggest that the biochar has low ash content and high surface area; thus, increasing pore volume and sorption sites.
![]() Equilibrium concentration (mg L−1)
Equilibrium concentration (mg L−1)
Figure 3. Sorption isotherms of atrazine by biochar, tire chips, steel slag, and limestone.
The high sorptivity of atrazine by biochars is influenced by their chemical properties. Biochar produced above 350˚C has lower H/C ratios and higher aromatic moieties than those pyrolyzed at lower temperatures [37] and its surface exhibits hydrophobic (aromatic moieties), acidic (carbonyl groups), and basic (pyrenes and chromenes) proper- ties due to the incomplete thermal decomposition and complexity of the feedstocks [38] . Accordingly, and considering the high atrazine sorption by biochar and their chemical properties; then, it is reasonable to suggest that several sorption mechanisms, including π-π interactions and hydrogen bonding, are involved. Several mechanisms have been shown to be operative in atrazine sorption to carbon including π-π interactions [39] , hydrogen bonding (as both bond acceptor and donor), and van der Waals interactions [40] .
The lower Kf value for the tire chips, compared to biochar, was not surprising given that biochar surface area was higher due to its smaller particle size and the chemical characteristics of both sorbents. In our study, 42% of the initial atrazine concentration was removed by tire chips during the sorption step (Table 1). From 83% and 96% of the atrazine initial concentrations (ranging from 0.5 to 7.5 mg∙L−1) were removed by rubber granules from waste tires and wood charcoal, respectively [41] . The differences in the size of the tire chips and type of material may have contributed to the lower sorption capacity in our study compared to the 0.15 - 0.30 mm size rubber granules used by Alam and coworkers [41] . In our study, the tire chips were a mix of different shapes and physical appearance and composition (Figure 1).
The calculated Freundlich sorption coefficients of atrazine sorption by limestone and steel slag were low (Table 1). These findings were not surprising giving the fact that these materials have poor affinity for organic compounds, including pesticides [42] . The affinity of atrazine to river sediments (with ~13% CaCO3) was attributed primarily to the organic matter in the sediments [43] . Likewise, soils with high carbonate content (>53% CaCO3) also showed low atrazine sorption [44] . Although blind inlets constructed with limestone bed material reduced the losses of atrazine and other pesticides, compared to the tile riser; this reduction was attributed to several factors, including reduced discharge rate, increased tortuosity, and the accumulation of sediments and soil organic carbon in the blind inlet bed [4] .
![]()
Table 1. Atrazine Freundlich sorption parameters for the media used in this study.
apH of the supernatant after the sorption step; bRegression coefficient between the experimental and the calculated data using the Freundlich equation; cPercent sorbed of the total atrazine added; Percent atrazine desorbed of the total sorbed; Net sorbed = Sorbed − Desorbed.
The lowest sorption capacity for atrazine was observed with the steel slag (Table 1). The steel slag derived from electric-arc-furnace steelmaking is composed mainly of Ca and Al oxides [23] . The extent of atrazine sorption by oxides is pH dependent, as pH decreases, atrazine sorption increases [40] . The solution pH was 9.6 after the sorption step when steel slag was used. When biochar was used as a sorbent, the pH solution was 8.6; yet, atrazine sorption was much greater than observed with steel slag (Table 1). These observations confirm the importance of materials with organic moieties, mainly those with hydrophobicity character such as biochar. At pH ranging from 4 to 8, atrazine sorption was insignificant by Fe oxides and smectites, whereas humic acid sorbed significant amounts of atrazine [45] .
3.3. Desorption Step
The percent atrazine desorbed by 0.01 M CaCl2 is presented in Table 1. No atrazine desorption was detected from biochar; whereas of the total atrazine sorbed 6% was desorbed from limestone, and 50% was desorbed from tire chips and steel slag. This suggested that atrazine was irreversible sorbed by biochar and moderately sorbed by tire chips. Biochar amendments to soils reduced weed control by atrazine up to 75% [46] , suggesting that biochar is an effective sorbent for atrazine when added to the soil. The low desorption from limestone, and perhaps steel slag, may have been due to the low overall amount of atrazine sorbed by these materials.
3.4. Atrazine Sorption by Dry and Pre-Wet Tire Chips
During drought periods, the blind inlet bed material may dry out; thus, a sorption-de- sorption study was conducted to assess the functionality of dried shredded tires to sorb atrazine. Compared to pre-wet shredded tires, 21% less sorption and 25% more desorption of atrazine (38% less net atrazine sorption) were observed with the dry tire chips than with tire chips pre-equilibrated with 0.01 M CaCl2. The sorption/desorption from this experiment for both dry and pre-wet conditions appears to follow the same trend, i.e. desorption after sorption was similar as perceived from plotting the sorption and desorption data (Figure 4). The mechanisms leading to the greater sorption/desorption behavior by the dry tire chips, compared to the pre-wet tire chips, is unknown. Atrazine may slowly diffuse into micropores and fissures in dry tire chips, and consequently, reduce sorption due to lack of a “pre-equilibrium step” before the atrazine addition. Slow diffusion processes, including “organic matter diffusion” and “sorption-retarded pore diffusion” describe slow sorption and desorption hysteresis of organic compounds into/from complex matrices [47] .
3.5. Sorbent Leaching Properties
The concentrations of leachates from the sorbents used in this experiment are listed in Table 2. The leaching potential of biochar was not evaluated since wood-based biochar usually does not contain large amounts of heavy metals of environmental concern [48] . The SPLP test is used to simulate the movement of inorganic phases under acidic
![]()
Table 2. Leachate concentrations from the isotherm sorption media using SPLC (Synthetic Precipitation Leaching Procedure) and TCLP (Toxicity Characteristic Leaching Procedure).
Valued in italic exceed USEPA drinking water threshold; BDL = Below Detection Limit; Analytes BDL: Ag, As, Ba, Be, Cd, Cu, Cr, Ni, Pb, Sb, Se, Sn, and Ti.
![]()
Figure 4. Atrazine sorption and desorption by the dry and pre-wet tire chips using initial atrazine concentrations of 50 and 100 μg∙L−1. (Filled symbols represent the sorption step, whereas the empty symbols represent the desorption step)
rainfall when the material is sitting in-situ [28] , whereas the TCLP test is used to simulate leaching of contaminants under landfill conditions. The SPLC and TCLP values were low or below the detection limit (BDL) of the ICP-EOS instrument. Eight metals (As, Ba, Cd, Cr, Pb, Hg, Se, and Ag) are listed by the U.S. EPA as hazardous, i.e. when concentrations exceed specific concentration levels, e.g. for Cd and Se is 1 mg∙L−1, and for Ba is 100 mg∙L−1 [49] . In our study, we analyzed for seven of these metals (except Hg) and their concentrations were BDL (<1.5 μg∙L−1); thus, the above results indicate that under the conditions of the SPLP and TCLP tests, the materials used in this study do not leach metals of environmental concern, and the concentrations do not exceed their hazardous levels.
Of interest, the TCLP extracted the transition metals Fe, Mn, and Zn with concentrations ranging from 4.01 to 8.76 mg∙L−1 from tire chip and steel slag samples (Table 2). Oxidized metal debris protruding in some of the tire chips was observed. Zinc, a component of the vulcanization process in the tire manufacturing [50] , has been detected in crumb rubber from recycled tires [51] . The TCLP test for steel slag yielded Fe and Mn (8.76 ± 1.76 and 4.01 ± 0.91 mg∙L−1, respectively). Visible incrustations of reddish oxides, likely Fe and Mn oxides were observed on the steel slag aggregates. In addition to transition metals, alkali and alkaline earth metals were observed in the limestone and steel slag extracts; where Ca was the highest, 67.3 and 209 mg∙L−1, respectively, followed by Mg and K (Table 2).
4. Summary and Conclusions
In this study, the sorption of atrazine by different sorbents was investigated. The sorbents used in this study were chosen as alternatives or add-on materials to limestone, the current blind inlet bed material, to remove atrazine in solution. The kinetic sorption data indicated that biochar used in this study removed atrazine faster than the tire chips, reaching equilibrium in 6 hours, compared to 30 hours for the tire chips. Furthermore, during a 24-hour sorption/desorption isotherm experiments, biochar was the most efficient material for removing atrazine (>99%), followed by tire chips (42%), limestone (<7%) and steel slag (<1%). The atrazine sorption mechanisms by biochar and tire chips were not investigated; however, hydrophobic interactions and H-bonding were likely mechanisms given the composition of these materials. The tests for leaching metals of environmental concerns from tire chips, steel slag, and limestone were negative, suggesting that these materials may be safe to use under the conditions of the SPLP and TCLP tests.
The information from this study may be useful in improving the effectiveness of conservation practices that require material to sorb pollutants of interest and filter sediments, e.g. blind inlets, filter socks, and rain gardens. Testing of conservation practices constructed using these materials should be conducted to determine if the sorption characteristics observed in the laboratory are operative under field conditions.
Acknowledgements
The authors would like to thank the staff at the NSERL, with special thanks to students and Janae Bos for the pesticide analysis.
Disclaimer
USDA is an equal opportunity provider and employer. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.