1. Introduction
Sporopollenin (SP), the biopolymer shell of pollen grains of higher plants, is a highly resilient yet poorly characterised material which has been described as “one of the most extraordinary resistant materials known in the organic world” [1] . Sporopollenin microcapsules have potential applications as drug carriers [1] , catalytic agent templates [2] , in masking the bad taste in the food industry [3] , and for waste water purification [4] [5] [6] . The unique surface morphology of these SP microcapsules enables them to be a good candidate for immobilisation of different materials such as enzymes and others. However, the complete chemical structure of the SP microparticles is still not fully documented. The outer layer, the exines, of the majority of pollen species has passed Raman spectroscopy analysis which was attributed to the high fluorescence background [7] . Sporopollenin has already been functionalised for applications in ion exchange and solid phase peptide synthesis [8] [9] . Mackenzie et al. [10] showed that sporopollenin derived from Lycopodium clavatum can be encapsulated with different organic materials such as vitamins, omega 3, enzymes and proteins. These different materials were loaded into the empty sporopollenin via either surface physical adsorption and/or the passive diffusion through the nano-channels (40 nm in diameter), existing on the SP surface, into the inside core of SP [11] . Paunov et al. [12] [13] demonstrated a new protocol for the encapsulation of living large cells such as yeast cells and different organic or inorganic materials into the exine of pollens from Lycopodium clavatum plants.
The paramagnetic iron oxide magnetite nanoparticles have attracted intriguing attentions in several environmental and biomedical applications owing to their biocompatibility [14] - [21] . They were usually surface modified with different functional groups in order to enhance their dispersion stability and affinity to different target species [14] [15] [16] . Immobilisation of biocompatible materials to these magnetic nanoparticles has provided a swift and safe separation of different biological molecules by utilising an external magnetic field [17] [18] [19] . Humic acid (HA), naturally originated by decomposition of mostly plant debris, is a fraction of the humic substances, which constitute around 50% - 80% of the natural organic matter in ground/surface water, sediments and soils [22] . Other known fractions of humic substances are fulvic acid (FA) and humin where all these three fractions are varied in their water solubility and apparent molecular weight. It was reported that HA has a high affinity towards different species due to its multifunctional structure (Scheme 1) that contains carboxyl, hydroxyl, carbonyl, amino and ether groups [23] [24] [25] . Despite the multifunctional feature of HA, its high solubility at pH > 2 in water makes it difficult to separate from suspensions and also restricts the use of HA as a solid substrate [26] . Therefore, it was necessary to immobilise HA to certain solid materials having special properties to take advantages of its unique features [27] . Recently, different types of materials, such as resins [26] , hematite [28] , alginate [29] , polymers [30] and silica particles [31] [32] [33] [34] [35] were used for HA capping. The interaction nature of HA and different metal cations in the environment is of substantial importance since the movement and transportation of these heavy metals in the environment are affected by the presence of the aforementioned
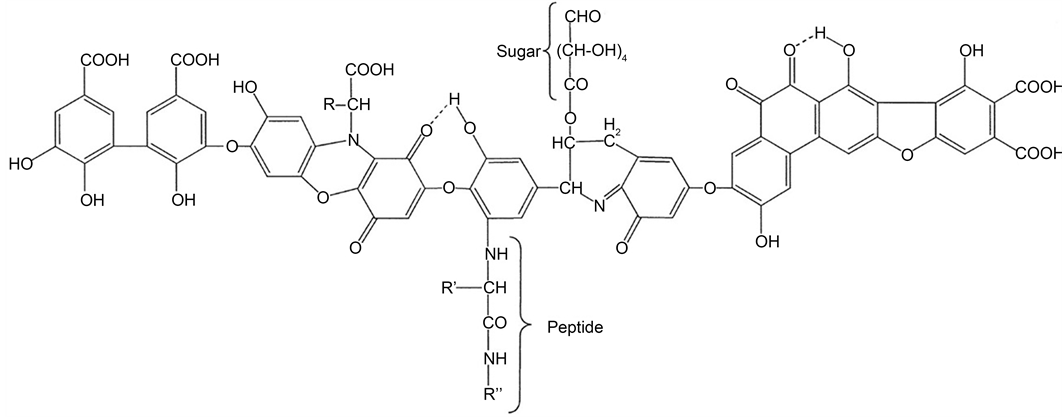
Scheme 1. Typical structure for Humic Acid (HA).
humic substances. The cumulative level of heavy metals (such as nickel, zinc, and cadmium) in water significantly affects the human health and environment [36] [37] .
Much effort has been devoted to developing efficient adsorbents with suitable chemical compositions, microstructures, and surface functionalities for the removal of heavy metals and organic pollutants from different aqueous media. The synergetic adsorption of heavy metals and humic substances is very challenging. This is mostly owing to their competitive adsorption onto most adsorbents. Therefore, the motivation of the current study was to develop suitable adsorbents having high adsorption capability, low toxicity and biocompatibility. Several materials, such as activated carbon, silica gel, resins, clays, functionalised sporopollenin, and biosorbents have been studied for the removal of heavy metal ions via sorption technique, which was extensively used [38] [39] . Metal sorption through precipitation and complexation is affected by the selection of suitable solid substrate which plays a vital role in mechanical, chemical, and thermal stability of the sorbents. Generally, studies which demonstrate the use of sporopollenin in complexation process are very few, although they all were based on chemical modification of SP surfaces [36] [37] [38] [39] . To the best of our knowledge, there exists no report on the use of sporopollenin from L. clavatum as support for in-situ encapsulation of HA-metal complexes or HA-magnetite metal complex nanoparticles without modification of the SP surface. In this study, we demonstrate different protocols to combine the multifunctional feature of HA, magnetic properties of iron oxide nanoparticles and low-cost and robust natural sporopollenin microcapsules through careful in-situ complexation, immobilisation and microencapsulation using these unique three biocompatible materials as depicted in Scheme 2.
2. Materials and Methods
2.1. Materials
Raw Lycopodium clavatum pollens powder was purchased from Fagron, UK. A stock
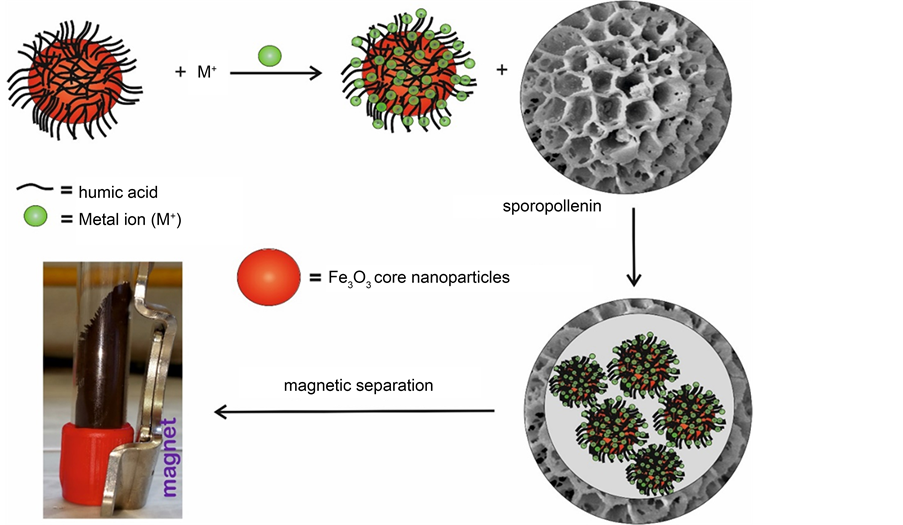
Scheme 2. Encapsulation of magnetic HA-metal complexes inside sporopollenin exines.
solution of 0.1 M solution of heavy metals ZnCl2, CdCl2, NiCl2, PbCl2 (purchased from Merck) was prepared by dissolving the appropriate amount of salt in double distilled water. Humic acid sodium salt having a molecular weight range of (2000 - 500,000), ferrous sulphate FeSO4∙7H2O, ferric chloride FeCl3, ammonium hydroxide (33%) and potassium iodide were purchased from Sigma-Aldrich. Double distilled water was used throughout all experiments.
2.2. Characterisations
The infrared spectra were obtained in the 650 - 4000 cm−1 range by a Perkin-Elmer 100 FTIR spectrometer. Samples were ground with anhydrous potassium bromide (spectrosol grade) to obtain disks to a ratio of 1/9 (w/w). FTIR spectra were a result of 3 scans against a background. Thermogravimetric analysis (TGA) curves were obtained on a Setaram TG Analyzer/Setsys analyser (EXSTAR S11 7300 at a temperature range of 298 - 1073). Transmission electron microscope (TEM) samples were prepared by dropping a diluted suspension of magnetite nanoparticles onto 400-mesh carbon-coated copper grids with the excessive solvent immediately evaporated using Hitachi H-800 TEM (Hitachi, Japan) at an operating voltage of 200 kV. Scanning electron microscope (SEM) analysis obtained using JSM-5400 LVJEOL (Japan). A platinum coating of was deposited on either empty or loaded SP samples by using an auto fine coater JFC-1600 (JEOL, Japan) at 20 mA for 1 minute. Images were taken with an acceleration voltage of 5 kV at various magnifications. X-ray diffraction (XRD) analysis was carried out using Philips X-ray diffractometer PW 1370; Co. Wrist Action. Metal ion concentrations in the solutions were measured using a flame atomic absorption spectrophotometer (Contr AA 300, Analytik je). Optical images were taken by Nikon microscope fitted with Lainsy digital camera (5 MP, Egypt) and the images were processed with microvision software.
2.3. Methods
2.3.1. Preparation of Sporopollenin
To take advantages of large cavity volume of the core of the spores, the core cytoplasm and the intine layer should be removed to extract the robust sporopollenin shell to be ready for encapsulation process. We extracted sporopollenin from Lycopodium clavatum pollen by suspending 100 g raw dry pollen powder in 800 mL acetone and stirred under reflux for 5 hours [12] . The de-fatted pollens were then filtered and 800 mL of 6 wt% KOH solution was added under reflux for 12 hours while the suspension was filtered and replaced once in the middle of the process. Then the suspension was filtered through Whatman filter paper 20 - 25 μm, washed with hot water followed by hot pure ethanol. The solid residue was suspended in 800 mL 85 wt% ortho-phosphoric acid, stirred under reflux until get rid of most of the inside cores of the raw spores. Then, the suspension was filtered off and washed with water, acetone, 2 M HCl acid and 2 M NaOH, water and acetone and finally washed with pure ethanol and then dried at 50˚C until constant weight.
2.3.2. Loading of Sporopollenin with Magnetite Nanoparticles
We have used two methods for preparation of magnetite nanoparticles: 1) the conventional method for co-precipitation of ferric and ferrous ions in alkaline medium and 2) the newly developed method by reacting only ferric ions as starting material and KI solution in alkaline medium [40] . The loading of SP microcapsules with different materials in this study was carried out either through passive diffusion through the SP shell or entering inside via opening the trilete scar by compressing the SP into tablets before use. The loading process was carried out as follows: The composition of solution A was as follows: 8.11 g FeCl3, 19.88 g FeSO4∙7H2O, 5 ml 5 M HCl, 40 ml distilled water. These solutions were mixed in a100 mL flask and heating to 50˚C until complete dissolution. Then 0.5 g of either the powder or a compressed tablet of sporopollenin was re-dispersed in 20 ml of this solution and stirred for around 2 hours at room temperature. The sporopollenin suspension was then filtered off and swiftly washed with distilled water, followed by an immediate transfer into a solution of 1 M ammonia (solution B). After 1.5 hours the obtained magnetic sporopollenin microcapsules, with entrapped magnetic nanoparticles MNPs, were filtered again and washed thoroughly with distilled water. Humic acid sodium salt solution was added subsequently with solution B to obtain HA-coated magnetite nanoparticles. In a previous work [40] , one of the authors succeeded to prepare magnetite and iron oxide nanoparticles using a simple method which only requires single iron salt as starting precursor, a small number of additional chemical reagents, and low process temperature lower than 50˚C. This method involves reacting a solution of anhydrous FeCl3 (40 g; in 300 mL of water) with KI (13.2 g, in 50 water) at room temperature. The reaction mixture was stirred for 1hr until complete precipitation of iodine. Then 35% ammonia solution (150 mL) was added slowly to the mixture at 55˚C. The reaction mixture was kept at this temperature for 3 hrs until complete formation of black magnetite nanoparticles precipitate. The precipitate was filtered, washed with distilled water, ethanol and dried to constant weight at 60˚C. This synthesis process was used to load empty SP with either bare or HA-coated magnetite nanoparticles for further metal uptake experiments. It is worth mentioning that the above describes protocol can be adapted to fabricate the magnetite nanoparticles inside the core of the SP microcapsules then attaching the HA-sodium salt to the formed nanoparticles taking into account the loading protocol used and the order of the addition of materials. It was reported that the adsorption of iodate anion on iron oxide nanoparticles affected the crystal growth of magnetite and produce more hydroxyl groups [40] . The formation of hydroxyl groups on the surface of iron oxide nanoparticles can enhance the coating process of the nanoparticles with biomolecules like HA or HA-metal complexes.
2.3.3. Preparation of HA-Metal Ion Complexes
Samples of HA-sodium salt (20 mg) were suspended in aqueous solutions (40 cm3) of the desired metal ion such as (Zn+2, Cd+2, Ni+2, Pb+2). The formed suspensions were mechanically stirred for 24 hrs at room temperature and the solid complex materials (referred to as HA-Zn for complexation with Zn+2) were separated by filtration and then lyophilised. The infrared spectra of HA, HA-Zn samples were measured in KBr pellets using FTIR spectrophotometer in the region 4000 - 400 cm−1 with the accumulation of 32 scans per sample.
3. Results and Discussion
3.1. Sporopollenin Characterisations
There are different protocols and methods that demonstrate the extraction of empty sporopollenin from their raw pollen grains. These extraction protocols can involve either harsh chemical treatments utilising strong acids and bases at raised temperatures or using mild conditions. It has been suggested that the hydrolysis of ester groups of the SP microparticles can occur under these harsh conditions resulted in a slight change to their structure [41] . Therefore, possible structure alternation has to be taken into account when one makes a comparison between empty sporopollenin microparticles originated from different plants species and produced through different extraction protocols.
Several analytical techniques have been used to completely determine the chemical structure of different sporopollenin species. However, their full chemical structure is yet incomplete and requires further studies. Nevertheless, some studies indicated that sporopollenin is mainly an aliphatic polymer with phenolic and aromatic groups or conjugated side chains [42] . It was also suggested that SP microparticles are macromolecules that essentially contain carotenoid and carotenoid esters [43] . For metal removal studies, many groups have appreciated the unique nature of the sporopollenin and have reported results with raw or surface functionalised form of sporopollenin. Therefore, SP can be considered a good candidate for those studies seeking more efficient, inexpensive and renewable adsorbents. In this respect, we report the advantages of using these important biocompatible materials for in-situ encapsulation of HA-metal complexes in addition to integrating a magnetic function to further manipulate the formed biosorbents.
In this section morphology of the surface sporopollenin after extracting the genetic materials from their cores is presented. Figures 1(a)-(d) show SEM images for the empty sporopollenin after the chemical extraction process from their raw pollens. It can be seen in the first place that the average particle size of the treated sporopollenin is around 27 μm, with nearly monodisperse distribution for this plant species with a rough surface which is one of the advantages of these natural particles. The native reticulate microstructure and ornamentation is retained for the treated SP particles as seen in the close-up SEM image in Figure 1(c), Figure 1(d), in addition, the trilete scars (Y shaped indicated by arrow) are also clearly visible in Figure 1(c) which is characteristic for this particular pollen species. Therefore, the chemically treated SP particles have intact surface morphology despite the relatively harsh treatment that they pass through during the extraction protocols used to obtain SP exines devoid from other core
![]()
Figure 1. (a)-(d) SEM image of empty sporopollenin exines from Lycopodium clavatum at different magnification showing the details structure morphology of the chemically extracted robust microcapsules. The reticulate microstructure and decoration on the surface are intact despite harsh chemical treatment to remove the cytoplasmic core and extract the SP exine shells. The average diameter of the sporopollenin capsules is 27 µm. The trilete scars on the SP surface are indicated by an arrow in (c).
components of the spore, including proteins, lipids, nucleic acids and polysaccharides. This indicative of the great resists of these SP against strong acids bases and several chemical attacks. Bohne et al. [44] investigated the structure of sporopollenin microcapsules prepared from pine pollen and concluded that the sporopollenin exine is perforated with nano-pores that make its membrane permeable. It is worth stress that loading these empty SP microparticles with the desired materials can be achieved either by passive diffusion of active materials through the nano-channels present on their surface and/or through the opening of the Y-shaped trilete scars (Figure 1(c)) via compressing the SP powder as a tablets (disk) before introducing to the solution in the correct step of loading to allow more materials to be entrapped inside the empty core ensuring an efficient encapsulation. That latter compressed tablet protocol was recently introduced by Paunov et al. [12] for encapsulating of large living cells into SP.
3.2. Characterisations of HA-Complex and Sporopollenin Microcapsules
3.2.1. FTIR for Pure HA and HA-Zn Complex without SP
Since humic substances contain several known functional groups such as carboxyl (-COOH), hydroxyl (-OH), amine (-NH2) and others, they exhibited great affinities to cations of different heavy metals. It was reported that the main functional groups found in HA (the main fraction of humic substances) are carboxyl, phenolic hydroxyls and alcoholic hydroxyls [45] . Therefore, we will focus on the most interesting FTIR bands of these main groups which might have a crucial role in the binding of metal ions. It was suggested that HA carries negative charges in weak acidic and basic media, which in turn promote the adsorption of cations via electrostatic interactions [45] [46] . In the current study we have used naturally originated HA-sodium salt (sodium humate) which originated from the results of the decomposition of organic matter, particularly dead plants and can be found in vegetable soil, peat, and/or soft coal. Recently, HA-Na has shown great performance in waste gas treatment, for example, in the absorption of SO2 and NO2 and other toxic gases particularly NO with the support of TiO2 nanoparticles [47] . Figure 2 represents FTIR analysis for pure HA-Na sample before interaction with either metal cations or magnetite nanoparticles. Significant peaks corresponding to the phenyl, hydroxyl, carboxyl, and the other substituents have been observed. The broad peak at 3431 cm−1 can be attributed to the phenolic -OH hydroxyl groups. The characteristic absorption bands of HA-Na are observed at 3693 cm−1 (stretching vibrations of -NH2), 1617 cm−1 and 1385 cm−1 (antisymmetric and symmetric COO- stretching vibrations of carboxylic salt), 1033 cm−1 (C-N stretching vibrations), 1010 cm−1 (C-O stretching vibrations in polysaccharides or polysaccharide-like substances) and 913 cm−1 (out-of-plane bending vibrations of aromatic CH groups). The double band at 2924 cm−1 can be attributed to aliphatic C-H bands.
It was reported that the origin of HA substances and their pre-treatments affects the sorption capacity of metal ions [48] . Figure 3 shows the FTIR of HA-Zn complex prepared by ion-exchange method. The two intensive bands can be observed between 1583 and 1384 cm−1. These absorptions may be assigned to symmetric (vsCOO−) and
![]()
Figure 2. FTIR for pure HA sodium salt showing its characteristic peaks. Important functional groups are carboxyl (-COOH) and hydroxyl (-OH) that show affinity to metal cations.
![]()
Figure 3. FTIR of HA-Zn complex prepared by ion-exchange method confirming the formation of the HA-Zn complex primarily via metal-carboxylate bonds.
antisymmetric (vasCOO−) stretching vibrations, respectively, of carboxylate groups, indicative that the HA-Zn complex of humic substances is formed primarily via metal-carboxylate bonds. The participation of other functional groups (phenolic hydroxyls, diketone groups) in the complexing of metals would be difficult to identify on the basis of FTIR analysis [49] . It was suggested that the calculated difference between the antisymmetric (vasCOO−) and symmetric (vsCOO−) carboxylate stretching frequencies of calcium- (195 cm−1), zinc- (200 cm−1) and copper-humate (215 cm−1), concluding that the metal-oxygen bonds are more covalent in the copper-humate than that in the zinc- and calcium-humates [49] . Figure 3 shows that the difference between these bands was (199 cm−1) which further confirms the successful formation of HA-Zn complex. It was reported that the most important characteristic of the FTIR spectra of HA substances is the presence of a peak at ~1700 cm−1 which is attributed to C=O stretching [50] . In addition, the interaction between the metal cation and carboxylic groups, to form a metal ion-complex, results in the disappearance of absorption bands at 1700 - 1720 cm−1 and the appearance of new bands at ~1583 cm−1 and 1384 cm−1 which assigned to stretching vsCOO− and vasCOO−, respectively. The disappearance of this band also suggests that most of the COOH groups were converted to the COO− form (Figure 3). We have also noticed that in Figure 3, the band at about 1583 cm−1 (vsCOO− stretching) becomes more and more intensive for HA-Zn complex, in contrast to the (vsCOO− stretching) band near 1617 cm−1 of pure HA-sodium salt (Figure 2) along with the shift to lower frequencies which also confirming the formation of HA-Zn complex.
It was reported that HA solutions with an initial pH > 7 are more suitable for formation of HA complexes than solutions having pH 7.0 [51] . In the alkaline medium the parent HA prefers complete dissociation of phenolic hydroxyl and carboxylic groups, results in an increase in the HA cation exchange capacity and ensure complete saturation of the HA molecule with Na+ ions as depicted in (Equation (1)) to form sodium humate (HA-Na) which was used in our current study for these reasons. According to soil chemistry, it is sensible to hypothesise that titration of such Na-humates with initially high pH would result in lowering the pH to neutral values towards the approaching of the equivalent point as a result of both cation exchange and salt formation as seen in (Equation (2)). These two reactions can be suggested as follows:
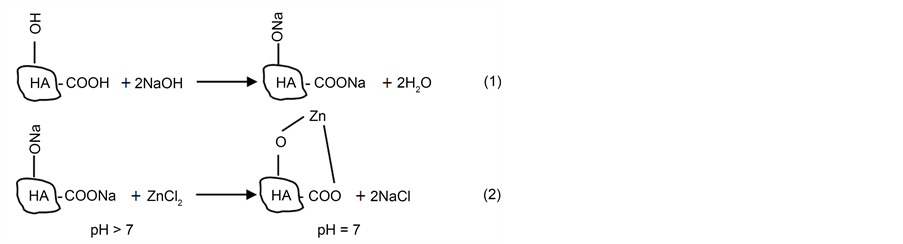
In line with the above interpretations, the use of neutral HA solutions will make phenolic OH groups unavailable for both dissociation and cation exchange. Therefore, the complex formation utilising the neutral HA solutions can be considered incomplete and takes place merely via electrovalent bonding by COO- groups [51] . We have conducted metal uptake experiments using HA-Na and found that higher metal recovery data (92%) were recorded for Zn++ from tap water at pH = 8 which is in agreement with the suggested mechanism shown in Equation (2). The full investigation of different metal ions sorption under different conditions using HA and/or magnetic SP-HA biosorbents is under way.
3.2.2. Sporopollenin Loaded with HA-Zn Complex
We demonstrate for the first time that metal-ion complexes can be encapsulated within the empty cores of the natural sporopollenin microparticles with or without biocompatible magnetite nanoparticles. This can be achieved using different encapsulation, complexation and capping protocols. As was mentioned earlier, active substances can be encapsulated into empty sporopollenin either via passive diffusion through their nano-channels or by diffusion through the trilete scar of these microparticles for those materials that are larger in size than the nano-channels [12] [13] . In this respect, we have used the latter protocol in which a compressed disk of empty sporopollenin powder was made and then quickly added to the metal-complex reaction in certain order, where we expected the formation of the HA-Zn complex would take place inside the cores of the sporopollenin after encapsulation of both sodium humate and Zn ions via their opened Y-shaped trilete. The resulted loaded SP have been collected washed, dried for FTIR analysis. It has been proved by Shaw et al. [52] [53] that the FTIR spectra of sporopollenin from different species are somewhat similar. Their work showed that sporopollenin contained hydroxyl groups, ethers, carbonyl groups and possibly the structure R-CO (R = aromatic, aliphatic or conjugation). It was suggested that the main structure of sporopollenin is a simple aliphatic polymer with aromatic and conjugated side groups. These studies also proposed that sporopollenin consists of a main structure or backbone with side chains that vary between species [53] .
The FTIR spectra of empty sporopollenin are shown in Figure 4 and the results are summarised in Table 1. Our results indicated that all our peaks are in agreement with the data obtained by other groups. Figure 5 represents FTIR spectra for sporopollenin encapsulated HA-Zn complex. Some new peaks can be seen comparing to the FTIR of
![]()
Figure 4. FTIR for empty Lycopodium clavatum sporopollenin microcapsules showing the characteristic peaks.
![]()
Table 1. FTIR data for empty Lycopodium clavatum sporopollenin.
![]()
Figure 5. FTIR for sporopollenin encapsulated HA-Zn complex confirming.
empty SP as shown in Figure 4. It can be clearly observed that the characteristic peaks of HA-Zn (Figure 3) are present in Figure 5 indicating the encapsulation of the HA-Zn complex within the sporopollenin cores.
In Figure 5, the N-H peak appears at 3693 cm−1 and the two new peaks at 470 - 540 cm−1 that occurred only in HA-Zn spectra. The broad O-H peak became more strong and the ether C-O peaks appeared more sharp and strong. Furthermore, the characteristic peaks of carboxylate group became sharp and strong compared with multiple peaks at (1500 - 1700 cm−1) of empty Sp as shown in Figure 4. It is worth mentioning that the presence of carboxylic groups in empty SP microparticles is somewhat controversial [52] [53] . No shift was observed for the SP characteristic bands at 2925 cm−1 and 2855 cm−1 (symmetric and asymmetric modes of CH2 group vibration) but the intensity of peaks has changed. From these data, it transpired that the contribution of the surface and/or core of SP microcapsules to the sorption of Zn ions could not easily be proved and requires more investigations.
The interactions of heavy metal ions with raw and surface modified sporopollenin have been previously studied [45] - [58] . However, all these results were based mostly on chemical immobilisation of different function groups to the surface of the SP to promote the sorption process, which involved several steps and reactions. In contrast to these reports, in our current study we demonstrated a much easier and greener way to uptake such toxic metals using natural biomaterials without chemical modification of empty SP.
Figures 6(a)-(d) shows optical and SEM images of sporopollenin suspension loaded with HA-Zn complex. The SP/HA-Zn suspension in pure water was observed using an optical microscope and the image is shown in Figure 6(a) where the rough surface of the encapsulated microparticles is clearly seen and the close size and intact shape of microparticles are evident. There was also no apparent aggregation behaviour for the loaded SP microparticles indicative of their reasonable colloidal stability when dispersed in pure water. Figures 6(b)-(d) represent the SEM morphology of the surface of
![]()
Figure 6. (a) An optical image for sporopollenin suspension loaded with HA-Zn complex showing the consistency of the size of SP microcapsules and the good dispersion in water without aggregations after loading process. (b)-(d) SEM images for sporopollenin loaded in situ with HA-Zn complex at different zooming magnifications confirming the intact structure morphology of the loaded microcapsules and the formation of metal-complex nanostructures on the SP surface (indicated by arrows in (d)).
the loaded SP microparticles and it can be clearly seen that the main surface decoration is intact after loading with HA-Zn complex. We noticed the formation of very small aggregations having a diameter of 1 μm or less which might be some of the formed HA-Zn complex nanoparticles deposited inside the surface network of the SP comparing to the clean surface of empty SP (Figure 1(d)). This is further confirming the incorporation of the complex with the SP.
3.2.3. Thermogravimetric Analysis
The thermogravimetric analysis (TGA) provides useful information on thermal stability and the amount of the materials encapsulated. The TGA of the empty and HA-Zn loaded Sp microcapsules are shown in Figure 7 and Figure 8 respectively. For the TGA of pure empty SP microparticles in Figure 7, the SP decomposed in three steps while the SP/HA-Zn microparticles decomposed in four steps (Figure 8). The empty sporopollenin started decomposition at 33.6˚C - 147.7˚C, which corresponds to a mass loss of 5.83%, followed by another loss of 45.6% in the 148˚C - 393.4˚C range, and finally a mass loss of 22.8% at 395.3˚C - 590.1˚C. The first step of this decomposition can be attributed to the release of water physically adsorbed on SP surface, while the second and third stages are due to the decomposition of organic material of SP to give the final residue of around 25.7%. The TGA data shown in Figure 8 for the HA-Zn loaded SP microparticles showed four decomposition steps, the first two steps ranging from 28˚C - 270˚C can be attributed to the loss of physically adsorbed water in SP and the metal complex. The third weight loss of 28.26% occurred in the 270˚C - 417˚C range which
![]()
Figure 7. TGA analysis for empty sporopollenin microcapsules showing three steps decomposition.
![]()
Figure 8. TGA analysis for sporopollenin loaded with HA-Zn complex showing four steps decomposition indicative of the successful loading process.
corresponds to the decomposition of both SP and HA organic materials. The fourth weight loss of 20.5% observed in 471˚C - 601.6˚C range can be attributed to the decomposition of remaining SP grains. It can be concluded from the TGA analysis of Figure 7 and Figure 8 that around 15% of residual HA-Zn remained for the loaded SP case indicative of the successful encapsulation of HA-Zn into the cavities of the empty SP microparticles.
3.2.4. Magnetic SP Loaded with HA-Zn Complex
We have further extended our investigation by attempting to encapsulate the magnetite coated HA into the SP biosorbents ensuring a greener way of uptake and recover the adsorbed heavy metal ions from different media. In this respect, different protocols can be used to achieve this new encapsulation green process. We have coated the magnetite nanoparticles first with HA-Na molecules then loaded them into the empty SP microparticles to be ready for metal ion uptake experiments. We have used two different methods for preparation of magnetite nanoparticles; the first was based on the classical co-precipitation of ferric and ferrous ions in basic medium and the second is a newly developed method based on the use only ferric ions as starting substance in a single reaction [40] . Owing to the exceptional magnetic properties, cost-effective synthesis and biocompatibility, magnetite Fe3O4 nanoparticles have been extensively used in numerous potential applications [40] [45] . Different magnetic iron oxide nanoparticles have been synthesised predominantly using the well-known co-precipitation method where a large amount of these unique materials can be obtained, that is why it is considered an advantageous method. However, the control over the particle size distribution is still inadequate, since the growth of the crystals is merely affected by kinetic factors [59] . In the current study, we have prepared magnetite nanoparticles using only one iron compound as precursor material using less number of additional chemicals and utilising simple reaction conditions [40] . The protocol of coating magnetite nanoparticles with HA sodium salt comprising the following steps: (a) An aqueous solution of FeCl3 was prepared; (b) then aqueous solution of KI was prepared; (c) the two solutions were mixed; (d) HA-Na and NH4OH solutions are then added sequentially at 50˚C by adjusting the pH to about 9 until we obtain the precipitate and (e) the precipitate was filtered off. The synthesis of magnetite Fe3O4 nanoparticles, prepared via this protocol, can be represented by the following equations [40] [60] :
 (3)
(3)
 (4)
(4)
It was anticipated that at pH ranging between 8 and 14 the complete precipitation of Fe3O4 would take place with a stoichiometric ratio of 2:1 (Fe3+/Fe2+) in oxygen free conditions according to the thermodynamics of the reaction represented in Equation (4) [59] . It was also proposed that the adsorption of iodate ions on the surfaces of the obtained magnetite nanoparticles could produce more -OH groups, therefore enhancing the sorption capacity of metal cations or other functional groups via covalent bonds. [40] . Figure 9(a) shows TEM image of the as-prepared HA-coated magnetite nanoparticles where the individual descript magnetite nanoparticles can be observed having an average diameter of 15 nm as revealed from TEM images analysis. The compressed disk of empty SP microparticles was then added to the suspension of magnetite/HA-Zn nanoparticles for encapsulation proves followed by the addition of the desired metal ion for complexation reaction. Figure 9(b) represents a digital image showing the
![]()
Figure 9. (a) TEM image of HA-coated magnetite nanoparticles prepared using KI method showing the individual nanoparticles and their diameters; (b) Magnetic susceptibility of the obtained sporopollenin microparticles encapsulated with magnetic HA-Zn complex for easy removal from the media after the metal or HA uptake process.
response of the magnetite/HA-Zn encapsulated SP microparticles biosorbents to the external magnetic field confirming the successful encapsulation of magnetite/HA-Zn nanoparticles into the SP. The tested uptake recovery of Zn++ ions from tap water using this new magnetic biosorbents was around 97%. Further and full sorption study is under way in our laboratory using these new solid natural materials.
To further confirm the successful coating of magnetite nanoparticles with HA-Na molecules, XRD and FTIR were performed for the coated magnetite. Figure 10(a) shows XRD for the HA-coated magnetite nanoparticles prepared using KI method where it can be concluded that the XRD diffractogram showed the pattern that can be related to the magnetite phase in accordance with the JCPDS powder diffraction document [PDF No. 65-3107]. Therefore, HA coating did not affect the crystal structure of the as-prepared magnetite nanoparticles. Figure 10(b) represents FTIR of the HA-coated magnetite nanoparticles prepared using KI method and this further confirms the attachment of HA to the Fe3O4 nanoparticles by appearing of the strong absorption peak at 590.57 cm−1, which corresponds to Fe-O stretching vibration [40] [59] . It was reported that the appearance of a new band at around 580 cm−1 (Fe-O stretching) without additional new bands indicated the formation of pure magnetite nanoparticles without other iron oxides [59] . These data confirmed the stability of prepared HA coated magnetite with KI method against environmental oxidation. We have noticed also significant differences in the bands assigned to C=O stretches (1401 and 1627 cm−1) which are consistent with the carboxylate anions interacting with the FeO surface as the C=O stretches in free carboxylate acids [61] . It transpired that the carboxylate groups might play an important role in the bonding of the HA to the magnetite surface. This result is in agreement with other authors [45] [61] . This proposed protocol can be applied to different metal-complexes, some metal oxides and legends to fabricate green natural functionalised materials.
4. Conclusion
We have shown that naturally, robust and low cost, sporopollenin exines microcapsules derived from the naturally occurring spores of Lycopodium clavatum can be loaded with HA-metal complexes or HA-coated magnetite nanoparticles for the first time. This has enabled us to take the advantages of utilising three green and biocompatible materials namely; sporopollenin, humic substances and magnetite nanoparticles. Humic acid substances showed high affinity to the metal ion studied with a high recovery percentage from tap water. The formation of the HA-Zn complex was confirmed using FTIR analysis and was successfully loaded into empty sporopollenin which also has been confirmed by FTIR and TGA analysis. Surface morphology of the SP before and after metal ion complex encapsulation did not show any significant change but some complexes nanostructures were observed inside the reticulate microstructure of the treated SP biosorbents. Interestingly, we showed also for the first time that biodegradable HA coated magnetite nanoparticles, prepared with KI via single ferric ion reaction method, can be encapsulated into empty sporopollenin. The resulted new biosorbents
![]()
Figure 10. (a) XRD and (b) FTIR pattern for HA-coated magnetite nanoparticles obtained by KI method confirming the successful capping of HA to the surface of the nanoparticles.
microcapsules can be used for enhanced magnetic removal of either heavy metals or HA substances from different aqueous media. Using different metal cations for complexation with other biologically functional molecules (as a model drug) inside the sporopollenin microcapsules can have a wide range potential clinical applications such as using metal cation-organic complexes as antimicrobial microcapsules. This allows the fabrication of benign encapsulates that would not release hazardous substances to the environment. We have demonstrated a proof of concept in this study; although, it can be applied to different metal-ion complexes, other metal oxide nanoparticles and different naturally occurring microcapsules which we believe will open up interesting analytical, pharmaceutical and medical applications.