Hydrochemical Characteristics of the Groundwater AQ1 of the Region from Pointe-Noire to Congo Brazzaville ()
1. Introduction
The groundwater is of a major importance in most areas of the world. However, this resource which was formerly of good quality, is presently threatened by various punctual and diffuse sources of contamination. The groundwaters constitute the main source of supply of the inhabitants of the area of Pointe-Noire. This area belongs to the coastal sedimentary basin, which extends on a surface from approximately 6000 km2 and is located in the south west part of the Congo. This coastal sedimentary basin presents a system hydrogeological to aquifer multi-layer ranging between 10 and 400 m from depth to Pointe-Noire and 70 and 150 m with the Indian Point. These aquifers are separated by intercalations from very composite materials (marly limestone, consolidated sandstones, clay…) of the reddish argilo sandy series of the greso-dolomitic and do not present a regular profile; the tender sands and sandstones are sandwiched between these layers. Water which it contains satisfies the water requirements drinkable, in industry and sylviculture. This water which constitutes an element impossible to circumvent for the economic development of the area is threatened of contamination. In spite of the importance of the economic and medical interests concerned, the operation of the multi-layer aquifer is still very badly known and the consequences of exponential and uncontrolled exploitation since the massive appearance of major drillings and traditional wells are not evaluated [1] . In the area of Pointe-Noire the only aquifers most exploited are AQ-1 and AQ2 (Figure 1). An investigation and observations on the ground show that aquifer AQ-1 is exploited by the population to supply itself with water via going wells of surface of depth from 0.50 to 10 m at the end of the dry season with an important seasonal variation going from 3 to 5 m. The exploitation of this aquifer being made in an artisanal way does not have precise data as for its evolution.
In spite of the identified threat and the economic, social and medical challenges that represent the aquiferous system of the coastal sedimentary basin of Pointe-Noire, the study and the management of this resource are not the objects of any program. Current knowledge relating only to the urban area of Pointe- Noire (and not the whole of the basin), comes essentially, from the research tasks of water [2] [3] , academics [1] [4] [5] which came to supplement those of the tankers. Since the end of 1980, the report of an intrusion of important salted bevel in the coastal accesses concentrated all the attentions of the public managers and the territorial collectivities.
It is thus advisable to know and follow the quality of this resource. The chemical composition of a water resulting from the natural environment is very variable. It depends on the geological nature of the ground from where it comes and also on the reactive substances which it could have met during the flow.
![]()
Figure 1. Hydrogeological cutting of the region of Pointe-Noire.
Underground water quality can be faded when external substances make contact with the aquifer. Such is the case of the even toxic undesirable substances which make groundwater unsuitable and toxic for various uses in particular for the use as drink water. The intensive use of the natural resources and the increase of human activities generate serious problems on the quality of the groundwaters [6] [7] .
The purpose of this hydrochemical study of the water of the hydrogeological AQ1 of the complex of the urban area of Pointe-Black is to identify the chemical facies of water, their degree of potability, as well as their aptitude for the overexploitation of this aquifer involving a probability of intrusion saltworks. It also makes it possible to follow the space-time evolution of the physicochemical parameters and to consider their mineral origin. Hydrogeological and physicochemical studies of groundwaters will be integrated and employed to determine the influence of these factors and the mechanisms controlling the chemistry of groundwaters in the area.
2. Materials and Methods
The physicochemical analyses were carried out on water of private wells of public use collecting the water table of the hydrogeological complex, including an aquiferous system composed of several aquifers superimposed with a hydraulic continuity, pertaining to the coastal sedimentary basin. This basin consists of a vast depression filled by a complex of plio-quaternary, tertiary and secondary deposits containing five aquiferous horizons or aquifers.
The samplings are carried out and conditioned in bottles especially prepared for this purpose. The samplings are carried out using an especially designed sampler. The device of sampling is carefully washed with the distilled water before each taking away. The near total of the water supply points of the aquifers is intended for the drinking water supply. To be used, water must meet certain standards which vary according to the type of use. The water samples intended for the chemical analyses were taken in eighteen (18) wells of the urban area of Pointe-Noire (Figure 2). For each test, physical parameters namely the pH, the temperature, and conductivity are measured in situ using a pH-meter and of a conductimeter of brand WTW 330.
The water samples were immediately stored with 4°C in a refrigerator containing the ice, the analysis was quickly carried out less 24H00 after the taking away. The major elements Calcium (Ca2+), Magnesium (Mg2+), Sodium (Na+), Potassium (K+), chloride (Cl), bicarbonate ( ), sulphate (
), sulphate (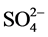 ) and hardness are analyzed in the laboratories of Civil engineering of the Higher National Polytechnic School (ENSP), the Research institute in Exact sciences and Natural (IRSEN) and of the National company of Water supply (SNDE). These analyses were carried out using a spectrophotometer by using the standardized classical methods.
) and hardness are analyzed in the laboratories of Civil engineering of the Higher National Polytechnic School (ENSP), the Research institute in Exact sciences and Natural (IRSEN) and of the National company of Water supply (SNDE). These analyses were carried out using a spectrophotometer by using the standardized classical methods.
The potability of water is defined by biological chemicaland physical parameters, but especially according to its use. A comparison of the contents of physical and chemical elements of water of various drillings to the standards of the World Health Organization was carried out according to [1] . The hydrochimical analysis was then carried out
![]()
Figure 2. Localization of the water samples taken for chemical analyses.
using the diagram of Piper in particular to characterize the geochemical facies of water of the AQ1 of the urban area of Pointe-Noire. This diagram is very frequently used and gives very good performances [1] [8] - [15] . The treatment was possible thanks to the software DIAGRAMS.
The second approach is based on the use of the various classical statistical analyses (average, quantile, median, standard, and the variance). The whole of the data collected on groundwaters of the area was the object of this statistical study. The statistical analysis has been carried out on 18 samples and 13 variables (electric conductivity (EC), the pH, the temperature (T˚C), hardness, TDS, Ca2+, Mg2+, Na+, K+, Cl, 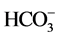 ,
, 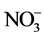 and
and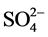 ) using the software RStudio. These various analysis make it possible to characterize the physicochemical aspects of the water of the water table of the AQ1 of the aquiferous system of the area of Pointe-Noire.
) using the software RStudio. These various analysis make it possible to characterize the physicochemical aspects of the water of the water table of the AQ1 of the aquiferous system of the area of Pointe-Noire.
The spatialization of the points of measurements was represented witch the help of the software Arc-Gis 8.2.
To assess the quality of the chemical analyses, the error of analysis on each water sample was evaluated starting from the formula (1) of the ionic balance:
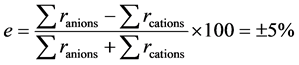 (1)
(1)
in which r represent the ionic concentration (meq/l).
3. Results and Discussion
For better understanding and better quantifying the variations of each studied parameter, only one representation of the data was used. This representation is based on the calculation of some statistical parameters, presenting the maximum, average, minimal values median, quantiles and the variances (Table 1 and Table 2). To facilitate the comparison and to better interpret the got results, we have in the same tables, the various maximum standards of potability of the water intended for human consumption according to the World Health Organization (WHO).
These synthesized analysis in various Table 1 and Table 2, show that the temperature of water of the AQ1 of the area of Pointe-Noire varies between 25.4˚C and 29.9˚C, with an average of 27.9˚C ± 1.0˚C. With regard to the pH of water of various points of measurement, it varies between 4.1 and 7.1 unities pH, for an average of 6.0 ± 0.6. Electric conductivity varies between 19 and 937.1 μS・cm−1, with a median value of 269.5 ± 243.1 μS・cm−1. Water with strong conductivity are those of the well of Mpaka (936 μS・cm−1), the well of Mbota (551.9 and 542.0 μS・cm−1), of the well of Tchiniambi (483.5 μS・cm−1) and of the well of Voungou (436.7 μS・cm−1).
The hardness of groundwaters of the zone of study varies between 0.0˚F and 11.0˚F, with an average of 3.2˚F. The water hardness is an indicator of the limestone level in water: it corresponds to its calcium content and magnesium. The more it contains some, the more it is “hard”. That reveals that water is in extreme cases soft and fairly hard as a whole. The water described as very hard is water of the well of Mpaka (11.0˚F), of the well of Voungou (7˚F) and of the wells of Mbota (5.0˚F). According to the guide
![]()
Table 1. Physic composition chemical average waters of the groundwater of the AQ1 of Pointe-Noire.
![]()
Table 2. Statistical analysis of the physic-chemical parameters.
of WHO, drinking of calcareous water or hard is not contra-indicated. Calcareous water takes part in the daily calcium contribution which the body needs (solidification of the bones, operation of the muscles, transmission of the nerve impulse, process of coagulation of blood). Water of the wells is characterized by the presence of the dissolved total solids which varies between 12 and 700 mg/l. Water is rich in sodium, with contents which vary between 2.4 and 161.9 mg/l, for an average of 38.8 ± 40.3 mg/l. The strongest value was obtained on the level of water of well of Mpaka (161.5 mg/l). The presence of sodium in water can be of anthropic origin or natural origin in the ground. Sodium can present dangers of encephalopathy for people to the chronic renal insufficiencies. The value of the guide of W.H.O of Total Sodium in the drink water is of 200 mg/l.
Water of the groundwater of the zone of study is rich in Ca2+, K+ and Mg2+ with a spatial variability which differs from a point of measurement with another, for respective averages of 8.0 ± 7.5; 3.3 ± 3.5 and 2.8 ± 2.1 mg/l. The ions sulphates (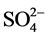 ) with concentrations higher to the value guides in the drink water can cause diarrhoeas in the human being [16] . The analysis of our data shows that the content of sulphate can be considered acceptable. The contamination by nitrates can be regarded as unacceptable with respect to the standard 50 mg/l for water of the wells of Mpaka, Mbota, Tchiniambi, Tchibati, Siafoumou, Tié-Tié, and Voungou (respectively: 302.4, 91.5, 65.8, 87.1, 103.9, 112.1 and 135.8 mg/l).
) with concentrations higher to the value guides in the drink water can cause diarrhoeas in the human being [16] . The analysis of our data shows that the content of sulphate can be considered acceptable. The contamination by nitrates can be regarded as unacceptable with respect to the standard 50 mg/l for water of the wells of Mpaka, Mbota, Tchiniambi, Tchibati, Siafoumou, Tié-Tié, and Voungou (respectively: 302.4, 91.5, 65.8, 87.1, 103.9, 112.1 and 135.8 mg/l).
The presence of nitrates testifies to a recent contamination resulting from the infiltration of waste waters and to a beginning of deficit of the environment in oxygen [17] [18] . The analyses of the water sampled in the area of Pointe-Noire show the values of the ions chlorides which vary between 1.9 and 90.9 mg/l, for an average the 26.3 ± 25.5 mg/l. Water of the wells of Mpaka, Mbota, Tchiniambi, Tchibati, Siafoumou, Tié-Tié, and Voungou present a rate of high chloride concentration. Chlorine is not dangerous for the man, but chlorination makes it possible to obtain an exempted water of virus starting from a water polluted by fecal organism when its concentration is maintained to the standards. The bicarbonate values in the zone of study are variable with a moyenne 24.2 ± 18.7 mg/l. Indeed, water being saturated or supersaturated with respect to calcite, a reduction in pH involves a bicarbonate increase.
Chemical facies of water of the free groundwater: The chemical data in major elements are represented in the diagram of Piper (Figure 3) which allows a representation of the anions and cations on two specific triangles whose sides express the relative contents (%) of each major ion compared to the total of these ions, expressed meq/l (cations: triangle of left, anions: triangle of right-hand side). The position of a water analysis in these two triangles makes it possible to specify which are the dominant anions and cations. The position of the analysis in the rhombus makes it possible to specify the total chemical facies [19] . It is important to stress that this diagram does not represent the total mineralization of a water, but only the distribution of the dissolved ions.
With each well a label of different colour and form was allotted. The diagram of Piper of the zone of study shows us the presence of 3 chemical facies including 12 samples having the facies chlorinated sodic and potassic either 67% of the total of the samples, 3
![]()
Figure 3. Classification of the ions from the mean annual Piper Diagram.
samples having the calcic and magnesian bicarbonated facies or 16.5% of the total and 3 samples present the sodic and potassic bicarbonated facies or 16.5% of the total (Figure 3). In the area of Pointe-Noire, water of the groundwater, is thus characterized by a prevalence of the ions chlorides on the ions bicarbonates and sulphates. Sodium constitutes the most important cation, then comes then Ca2+. Only wells PU8, PU3 and PU5 do not present dominant cations (Figure 4). From the diagram of Schöeller Berkaloff (Figure 5), we represented each analysis by a broken line, characteristic profile of the concentration of each major ion in solution in water, the six logarithmic scales at equal distance from/to each other, being shifted in order to align the unit values of the milliequivalents of various anions and cations. These profiles highlight the relationship of water between themselves, which show the presence of the three sodic and potassic chlorinated facies (in large the majority of the wells), bicarbonated sodic and potassic then the calcic and magnesian facies bicarbonated for water of the free groundwater of the area of Pointe-Noire.
This prevalence of the sodic and potassic facies chlorinated is shown through the diagram of Stiff (Figure 6). By analyzing the diagram of Stiff, onenotices the predominance of the cations Na+, and K+ in the majority of the wells and of the anions 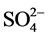
![]()
Figure 4. Classification of the ions from the Stabler Diagram.
and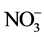 , this one is due to the intrusion salt works and/or the immediate anthropic pollution of the wells in unconfined water AQ1.
, this one is due to the intrusion salt works and/or the immediate anthropic pollution of the wells in unconfined water AQ1.
The diagram of Wilcox (Figure 7) shows that the majority of the well collecting the unconfined water of the area of Pointe-Noire present an excellent water. Some wells such as: PU1, PU2, PU3, PU4, PU5 and PU16 present a more or less acceptable water. The calculation of relationship between chemical elements makes it possible to high- light the mixture of water of different origins. Several studies ( [1] [20] - [23] ) give exam- ples of use of reports to identify the origin of the salinisation of water such as Cl−/Br,
Na+/Cl−, Na+/K+, Ca2+/Mg2+, 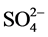 /Cl−… These reports can also be represented in the form of graphs of an element compared to the other.
/Cl−… These reports can also be represented in the form of graphs of an element compared to the other.
The hydrochimy of the free groundwater is also characterized by various ionic reports (Table 3). The Ca/Mg report is characteristic of the course of groundwaters, variable between 0.27 and 73.54 (Figure 8(a)). The strongest values are observed in water of well PU16.This could be explained by a more important time of residence at the level of this well.
The report Cl/Na (Figure 8(b)) lies between 0.22 and 1.04. If sodium would come from the dissolution of halite, this report should be equal to 1. However it is lower at 1 in 90.90% of the water samples, which indicates that the primary source of sodium- comes from salts such as the halite or sulphate of sodium, nor of potassium chloride or potassium sulphate.
4. Conclusion
The study of the hydrochemical characteristics of undergroundwaters of the unconfined water of the area of Pointe-Noire was carried out starting from the combination of the hydrogeological, hydrochemical methods, and of the classical statistical analysis. This study highlights the various physicochemical characteristics of water of the site. The study raises that the undergroundwaters are in extreme cases of an intrusion salt-
![]() (a)
(a)![]() (b)
(b)
Figure 8. (a) Report Ca/Mg; (b) Report Cl/Na.
![]()
Table 3. Ionic reports and chemical facies of the samples of waters.
works, because the limits of the diagram of Wilcox show an excellent water in the majority of the wells which tend towards the limit of admissibility. This hydrochemical study made it possible to highlight the prevalence of sodic and potassic facies chlorinated in groundwaters of the AQ1 unconfined water in the area of Pointe-Noire. In this area, the groundwaters of the unconfined water gather in three principal-hydro facies: Potassic and sodic chlorinated water is the most important (66.7%) studied water; calcic and magnesian bicarbonated water (16.65%) and the potassic and sodic bicarbonate ones (16.65%).