Effect of Morphological and Physiological Development on the Acclimatization of in Vitro Plants of Bambusa vulgaris Schrad ex Wendl in Liquid Culture Medium ()

Subject Areas: Biotechnology, Environmental Sciences, Plant Science
1. Introduction
In Cuba, B. vulgaris (Bambusa vulgaris Schrad. ex Wendl) has been promoted in order to solve the deforested environments and economic problems [1] . However, plant micropropagation is one of the most promising methods for developing large-scale production of many crops, such as bamboo species. This method is a favorable alternative to increase the number of in vitro plant of B. vulgaris with high uniformity within a shorter period of time [2] .
However, the use of semisolid culture medium during plants formation is still a constraint [3] . Under these conditions, the physical state of the culture medium, the high relative humidity and CO2 concentration inside a jar (because of reduced gas exchange) may influence anatomical, physiological and morphological plant characteristics, and can result in great losses during acclimatization [4] . These difficulties can be overcome by using liquid culture medium [5] .
Using liquid media in micropropagation processes is considered as the ideal solution for reducing plantlet production costs and enabling automation [6] . Various procedures have been developed to improve growth and the morpho- physiological characteristics in vitro plants [7] .
The goal of this study was to analyze the effect of morphological development on the acclimatization of in vitro plants of B. vulgaris in liquid culture medium.
2. Materials and Methods
2.1. Plant Material and Culture Conditions
Axillary buds (average 2 cm) were excised from plants of B. vulgaris pre-existing in greenhouse. The explants were dipped in 70% alcohol for 3 s, then surface-disinfected in 2% sodium hypochlorite solution (NaClO) for 20 min, and then rinsed three times with sterile distilled water. These explants were inoculated in glass tubes jars (20 cm high 1, 5 cm internal diameter) containing 10 mL of basal MS medium [8] , supplemented with 6.0 µM BA (100 mg∙L−1 myo-inositol and 30 g∙L−1 sucrose. The cultures were incubated at 25˚C ± 2˚C with 16 h light (fluorescent lamps with photon lux light intensity of 40 lmol∙m−2∙s−1).
2.2. Shoot Multiplication
After 20 days, the shoots were inoculated in polycarbonate magenta (250 mL), containing 70 mL in basal MS proliferation liquid culture medium (8) supplemented with 6.0 µM BA, myo-inositol (100 mg∙L−1) and 30 g∙L−1 sucrose.
Two methodologies were studied to multiply the shoots. In one of them, single-node explants were dissected from the shoots growing in vitro when they reached 7 - 10 cm in height, and placed on fresh medium, in a similar manner to the original explants. In the other, large clumps (with 8 - 10 shoots), obtained from growth of lateral buds, were divided in smaller clumps, (3 - 5 shoots each), placed on the same culture medium. The cultures were incubated at 25˚C ± 2˚C with 16 h light (fluorescent lamps with photon lux light intensity of 40 lmol∙m−2∙s−1).
The multiplication rate (MR) was calculated as:
MR = total number of shoots per plants/initial number of shoots per plants.
2.3. Plant Acclimatization
After 30 days in culture, the in vitro plantlets were transferred to a greenhouse. These plants were then planted in black polyethylene containers (5 cm × 10 cm) containing a mixture of organic matter (humus and Zeolite 1:1) mixture. Once planted, the plants were incubated at 30 ± 20˚C, RH 90% and then maintained under 50% shade with intermittent-mist water sprays to avoid damage due to desiccation and maintained for 90 days.
Two heights 3.0 cm (G3) and 4.0 cm (G4) were studied in vitro plants of B. vulgaris on greenhouse..
Morphological and Physiological parameters
The roots number/plants, leaf number/plants, fresh and dry weight t (FW and DW) (g∙plant−1), water content (WC), leaf area (cm2∙plant−1), net assimilation rate (NAR) and leaf area index (LAI ) were assessed after 30, 60 and 90 days after planting
From the above determinations, the following physiological parameters were calculated:
Water content (WC)
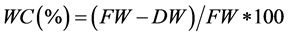
Leaf area
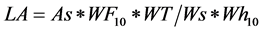
LA: Total Leaf Area plant; As: Area of a square Paper 1 dm2; Ws: weight paper square 1 dm2; WF10: Weight ten paper figures; WT: Fresh weight (g) of all the leaflets of the plant; Wh10: Fresh weight (g) ten leaflets of the plant.
Leaf area index
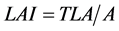
LAI: Leaf area index; TLA: Total Leaf Area of the plant; A: Vital plant area
Net assimilation rate
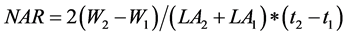
NAR: Net assimilation rate; W1: Initial weight of the total dry matter (g); W2: Final weight of the total dry matter (g); LA2: Final Leaf Area; LA1: Initial Leaf Area; t1: Initial time; t2: End time.
2.4. Statistical Analysis
Data were analysed using SPSS version 18 for Windows. Normality of data was tested using the Kolmogorov- Smirnov test. Significance of differences was determined by analysis of variance (ANOVA), and Dunnett’s test at 5% differences among the mean values.
3. Results
3.1. Plant Material and Culture Conditions
The liquid medium stimulates the shoots proliferation after 20 days culture, without the contaminants presence (Figure 1(a)).
3.2. Shoot Multiplication
The single nodal segments do not produce lateral shoots. Nevertheless, the explants in a unit of three shoots responded better than the individual shoots, developed several shoots (Figure 1(c)). In addition, the multiplication rate increased (3.0) after two weeks (Figure 2).
The results showed, that liquid culture medium is an important factor for to in vitro multiplication of bamboo [9] . Then again, a significant correlation was observed between the new shoots and the number of plants in greenhouse (Figure 1(e)).
3.3. Ex Vitro Rooting and Acclimatization
The simple ANOVA showed that G4 increased the ex vitro survival. This treatment produced the greatest percentage of root, number of leaves per plants. Furthermore, the highest water content, leaf area, fresh and dry weight, was found in similarly plants (Figure 3).
The observations of net assimilation rate and leaf area index showed a significant variation in vitro plant of B. vulgaris under ex vitro conditions. As well, there was a significant interaction between in vitro plants and different growth parameters. The G3 had the highest physiological indices, whereas the lowest value was observed in G4 (Figure 4).
![]()
Figure 2. Growth of shoots of Bambusa vulgaris at in vitro multiplication. y = 0.84 + 0.51x + 0.02x2.
4. Discussion
In the present study, we evaluated the physiological response of B. vulgaris during acclimatization phase. The plants analysed in this work exhibited a diverse range of morphological and physiological responses. The growth also had a significant effect on development of the plants. These observations are consistent with earlier reports on different plant species [10] - [14] , which showed that plants in greenhouse had different growth characteristics.
Our results on survival percentages of plants were demonstrated that liquid culture medium has a direct bearing on success in the hardening process and further acclimation of in vitro plants of B. vulgaris. Similar observations in other crops have been described earlier [15] - [19] .
The net assimilation rate and leaf area index curves (Figure 4) indicate that G3 and G4 have similar physio-
logical parameters. The water content, leaf area, fresh and dry weight increased in parallel by increasing the height (Figures 3(c)-(f)).
The visual assessments (data not shown) of the plants under ex vitro conditions demonstrate that the G4 had longer and thinner leaves than the G3. Similarly, the B. vulgaris had pronounced differences with regard to their morphology and, in particular, their leaf characteristics. G4 had long and thin leaves, and G3 had shorter and broader leaves. Similarly, G3 had a stronger reduction in the leaf water content (Figure 3(f)).
In our study, point morpho-physiological measurements confirmed the increase of leaf area, fresh and dry weight (Figures 3(c)-(f)). The parallel increased in the growth characteristics of development of in vitro grown plants of B. vulgaris (Figure 3) also contributed for an increase of the physiological parameters (Figure 4).
The changes in leaf width (Figure 1(c)), which were associated with leaf shrinkage and leaf rolling, are caused by the loss of water particularly from the bulliform cells located above the midrib as a protective mechanism in monocots to avoid further water loss [20] .
The results reported here reinforce other reports in the literature on the effects of morphological development on the acclimatization of in vitro plants in morphogenesis in vitro, and widen the possibilities of using alternative and efficient materials to promote in vitro growth of plants by increasing during in vitro propagation.
![]()
![]() (a) (b)Bars with different letters differ significantly by Dunnett C at P\0.05.
(a) (b)Bars with different letters differ significantly by Dunnett C at P\0.05.
Figure 4. Physiological parameters related to growth of in vitro plants of B. vulgaris under ex vitro conditions. (a) Net assimilation rate; (b) Leaf area index. *Each value is the mean for 80 shoots ± average range (standard error) of the mean.
Thus, the liquid culture medium has improved the morphological and physiological characteristics such as proliferation rate, net assimilation rate and leaf area index, making it a possible alternative for in vitro commercial propagation systems that are based on physiological growth patterns.
5. Conclusion
In conclusion, the report describes the positive effect of the morphological and physiological parameters on in vitro propagation of B. vulgaris. During the acclimatization phase to ex vitro conditions, the in vitro plants showed 75% survival. Results showed that greater development of the root system, height and number of leaves per plants was observed with height larger of 3.0 cm. The leaf area, water content, fresh and dry weight increased with height larger of 3.0 cm, but all parameters were decreased with height (1.0 - 3.0 cm). The correlation between net assimilation rate and leaf area index was significantly positive in vitro plants with height larger of 3.0 cm. Results obtained in the present study clearly demonstrated that liquid culture medium had a definite influence on plant growth parameters, anatomical features of leaf and in turn survival rate of plants raised under ex vitro conditions. These features are necessary for the adoption of in vitro propagation technology for large-scale multiplication of this species. The outcome of the work would help in refining the multiplication and acclimatization steps for the development of technology packages for mass-scale propagation and cultivation of B. vulgaris, this will not only help in large- scale multiplication of this useful multipurpose bamboo species for the restoration of degraded land, but also result in deriving economic benefits for the local communities.