Worsening Clinical Outcome with Increasing Number of So-Called Off-Label or Unapproved Indications for Use of Drug Eluting Stents ()
Received 31 May 2016; accepted 9 July 2016; published 12 July 2016

1. Introduction
New stent brands are continually developed in order to improve stent performance and clinical outcome. The improvement is obtained by changes in several factors in metal alloy, manufacturing technique, strut architecture, coating material, drug type and amount, medication release kinetics and stent balloon delivery systems.
The clinical outcome is not only determined by differences in these factors. It is also dependent on several patient and lesion characteristics like the so called “off-label” or unapproved indications for use of drug eluting. One stent brand may be particularly good when some off-label characteristics are present, but it may also be particularly bad in other cases. If the particularly bad cases for use of one stent brand could be identified then another and better stent could be selected and the clinical outcome for the population might thereby be improved without even manufacturing a new stent.
We explored the impact of increasing number of off-label lesions and patient characteristics on 5-year clinical outcome following implantation of first generation drug eluting stents. Furthermore we tried to assess the impact on outcome for each identifiable patient or lesion characteristic for each stent type. Finally, we attempted an estimation of the effect on outcome if the off-label characteristics present were taken into account to guide the stent brand selection.
2. Material
In the SORT OUT II trial 2098 patients with 2888 lesions were randomized to sirolimus- or paclitaxel-eluting stent implantation. The 18 months results and the 5 years results have been published [1] [2] . All patients were followed until emigration, death or five years of clinical observation. The SORT OUT II was the largest of the randomized studies that compared these first-generation drug-eluting stents [3] . The SORT OUT II trial was especially designed to reflect daily routine in clinical practice by including unselected all-comer patients and avoid preplanned repeat angiography. One third of the patients are treated for more than one lesion and most of the lesions have more than one off-label characteristic (Table 1) [1] .
3. Methods
When off-label characteristics are used to guide stent brand selection it may be defined as an individualized stent brand selection approach. This is opposed to a so-called general stent brand selection approach in which one stent brand is used in all patients.
First we compared the two stent brands with the MACE rates for the 2888 lesions treated with sirolimus- or paclitaxel-eluting stents.
Second, we searched for a possible relation between the numbers of concomitantly appearing off-label characteristics and the MACE rate.
![]()
![]()
Table 1. Uniform definitions of on- and off-label characteristics for the use of a drug eluting stent.
Third, we investigated the impact of each off-label characteristic on MACE rates for each stent brand. This was estimated by a hazard ratio (HR) for MACE when off-label characteristics were present in proportion to zero off-label characteristics present.
Finally, we estimated the magnitude of the potential effect of an individualized stent brand selection approach. The optimal stent to use would be the stent brand with the lowest HR for MACE when HRsirolimus is compared to HRpaclitaxel for each individual lesion.
Statistical Methods
All analyses were done according to the intention to treat principle and performed by use of SPSS 17.0 statistical package [1] . The different subgroups had different numbers of patients or lesions. The statistical power to detect different effects on MACE varied accordingly. The subgroup sizes were not necessarily large enough to obtain power to determine whether any characteristic was of statistical significance. Consequently, our backward stepwise multivariate Cox regression analysis was not based on statistical significance. Instead we included off label characteristics that was correlated to a relative change in hazard for MACE of at least 25% (i.e. a HR of ≥1.25 or ≤0.8). Afterwards we assessed the validity of the result by use of conventional stepwise regression now based upon significance levels.
Multivariate Cox regression analysis equations were developed for MACE. For each lesion we calculated the HR connected to the use of either the paclitaxel-eluting (HRpaclitaxel) or the sirolimus-eluting (HRsirolimus) stent brand for the specific off-label combination. First all 18 off-labels were forced into the model. Then we identified that off-label characteristic with least clinical importance (identified by a HR closest to 1.0). This off-label characteristic was removed. The remaining 17 off-labels were forced into the model and a new off label closest to 1.0 was identified and removed. This stepwise regression was repeated until all HR were outside 0.8 - 1.25. The model was tested for statistically significant interaction by a correlation regression table. The assumption of constant proportional hazard was tested by log minus log plots. The same method was repeated in the group of patients with only one lesion in order to assure independent variables in the equations. Thereby the HR obtained in the total group of lesions could be checked for discrepancies.
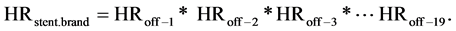
The equation above contain to the left a HRstent.brand being a proportional hazard with the hazard for MACE for a lesion with some off-label characteristics in the numerator divided by the hazard for MACE for a lesion with zero off-label characteristics in the denominator: If an off-label characteristic is not present the corresponding HRoff-number is replaced by the factor 1. By calculating the HR stent.brand for a specific off-label combination for each stent brand two different HR’s may be found. By example HRpaclitaxel = 1.20 and HRsirolimus = 1.60. The two HR cannot be directly compared because the sirolimus eluting stent appears to hold a MACE rate of 90% of that of the paclitaxel eluting stent groups. Consequently the sirolimus eluting stent may possess a HRsirolimus that is a fraction higher than the HRpaclitaxel and still be the better stent brand choice. A simple way to enable direct comparison is to multiply the HRsirolimus by 0.9 and achieve a corrected HRsirolimus = 1.60 × 0.9 = 1.44. In this example the paclitaxel eluting stent should be selected to use in that specific lesion because 1.20 is lower than 1.44.
These HR’s were calculated from the Cox regression equations on off-label characteristics with an impact on MACE of at least 25%. The lesions treated by stent brands with the “lowest or at least equal” HR are defined to have been treated with the optimal stent brand. Lesions treated by stent brands with highest HR are defined to have been treated with the worst stent brand. The MACE rate for lesions treated by the optimal stent was compared to the MACE rate for the sirolimus eluting stent group. This stent was chosen because a meta-analysis has found the sirolimus eluting stent to be significantly better than the paclitaxel eluting stent [3] . The comparison would reflect the effect of an individualized stent brand selection approach versus a generalized stent brand selection approach.
4. Results
A total of 1065 patients with 1481 treated lesions were randomized to sirolimus-eluting and 1033 patients with 1407 treated lesions to paclitaxel-eluting stent implantation. The five year MACE rate was 20.8% for lesions treated by a sirolimus-eluting stents and was 22.9% for lesions treated by a paclitaxel-eluting stents (log rank test, HR 0.90, 95% confidence interval 0.77 - 1.05, Chi square 1.77, p = 0.18, Figure 1).
In lesions with zero off-label characteristics we found a MACE rate of 16.7%. With an increasing number of off-label characteristics in each lesion we found a statistically significant stepwise increase in MACE rate up to 32.7% in lesions with 5 off-label characteristics (log rank test for trend, Chi square = 44.32, df = 1, p < 0.0001, Figure 2). The HR’s for MACE was estimated for all off-label characteristics as well as for the off-label characteristics with an impact on MACE of at least 25% (HR outside 0.8 - 1.25, Table 2). According to the study randomization a lesion could be classified to having been treated either by the optimal stent with the “lowest or at least equal HR” or by the worst stent with the “highest HR”. By comparison between those two groups the optimal stent group had a HR for MACE of 0.74 compared to the worst stent group (log rank test, Chi square 14.42, p = 0.0001, df = 1, 95% confidence interval 0.63 - 0.86). The MACE rate for the optimal stent group was 18.8% compared to 20.8% for the sirolimus treated lesions equal to a HR of 0.90 (log rank test, Chi square 1.75, p = 0.20, df = 1, 95% confidence interval 0.76 - 1.06).
The sum of all HRpaclitaxel and 0.9* HRsirolimus according to the randomization amounted to 4266 and corresponded to 628 MACE. Those lesions that happened to be treated by the worst stent brand with a higher HR might have had a lower MACE rate if treated by the alternative stent. If the HR for these lesions were replaced by the HR for the alternative and better stent then the virtual sum of HRpaclitaxel and 0.9* HRsirolimus would have been 3500. This sum is 0.82 times the above mentioned 4266 corresponding to an expected MACE rate of 515 instead of the real MACE rate of 628. This risk reduction would be to a value of 0.82.
5. Discussion
The comparison of MACE rates between the two stent brands showed as expected nearly the same result using a lesion perspective (n = 2888) as with a patient perspective (n = 2098) [2] .
![]()
Figure 1. Cumulative proportions of lesions experiencing major adverse cardiac events during 5 years of follow up if treated by sirolimus(cypher)- or paclitaxel(taxus)-eluting stents. Abscissa: days. Ordinate: percentage.
![]()
Figure 2. Cumulative proportions of lesions experiencing Major Adverse Cardiac Events during 5 years of follow up when grouped by Zero (n = 138), One (n = 549), Two (n = 753), Three (n = 627) and Four (n = 427) or Five (n = 223) concomitantly appearing off-label characteristics in each lesion. Abscissa: days. Ordinate: percentage.
In the present study of patients randomized in the SORT OUT II to one of the two first generation drug- eluting stents, we used 18 different off-label characteristics to show that MACE rates increased with an increasing number of off-label characteristics. This was also expected and has now been proven. The fact that the multiple stepwise regression analysis found some off-label characteristics connected to a HR < 1.0 may explain why the MACE curves for zero, 1, 2, 3, 4 and 5 concomitantly appearing off label characteristics are not just stacked lines in numerical order (Figure 2).
For each stent brand the HR connected to each off-label characteristics has now been estimated with up to 18 different off-label characteristics. This result is only applicable to the presently investigated stents and our Cox regression equations for the sirolimus- and the paclitaxel-eluting stent need further confirmation in other studies. These other studies have hardly tracked the same number or type of off-label characteristics as we have, and the extraction of the clinically most important ones (HR outside 0.8 - 1.25) are therefore specifically justified.
Multiple Cox regression analysis necessitates a lot of observations and a very large database to extract the equations from. If such a large database is non-existing it will be impossible to execute a stepwise exclusion based on statistical significance. But as sufficient data is often scarce, our way of selecting the variables after clinical importance in preference to statistical significance is a way to identify those parameters with important impact on health. Of course, statistical significance is the preferred method but in the present case it would have been at the cost of missed identification of characteristics of importance to clinical outcome, because the present study―despite its size―was not powered to investigate all subgroups. Our threshold for inclusion of a characteristic into the equations requires an impact on HR of at least 25% in MACE. It is reassuring, that 14 of the 18
![]()
Table 2. The impact on MACE from off-label use. First column is the off-label number. Second column is the description of the off-label item grouped for each stent brand. Third column is the hazard ration (HR) for MACE with all 18 off-label characteristics forced into the model. Fourth and fifth columns contain the 95% confidence interval (CI) for the HR. Sixth column contains the p-value for the HR. The last columns are repetitions of column three through six but now only with the off-label characteristics that have an effect on MACE of at least 25% (HR outside the range 0.80 - 1.25).
included characteristics (14 of 18) in the two regression equations were in fact of statistical significance. Even smaller effects on MACE may be justified but it takes more observations than what the present investigation contain. A meta-analysis has found the sirolimus eluting stent to be significantly better than the paclitaxel eluting stent [3] . The sirolimus-eluting stent in our study was slightly superior with a relative MACE rate of 0.90 compared to that of the paclitaxel-eluting stent. Even though that result was ns the meta-analysis result justified that the HR concerning sirolimus-eluting stent should be multiplied by 0.9 to enable translation to a direct comparison of the expected hazard for MACE between the two stents. It was then possible to determine whether the initially randomized stent in fact happened to be the worst stent (posses the highest HR) or the presumed optimal stent (lower or at least equal HR).
Compared to a general use of only one stent brand the MACE rates may be reduced by 10% - 18% if the combination of off-label characteristics was used to guide stent selection in each individual case. Such effect size may be worth looking for in newer generation drug eluting stents.
Clinical Perspective of the Individualized Stent Brand Selection Approach
As drug-eluting stents are approaching maximal obtainable stent performance any new invention or improvement can only induce modest changes in clinical outcome. The traditional approach is to identify the best stent to use in a population―which unfortunately will include cases where an alternative stent would have been a better choice. Opposed to this general approach is an individualized stent brand selection approach were each individual patient’s and lesions off-label characteristics are important and are used to determine which stent brand to select. Such an individualized approach may help to ensure, that we are in fact treating each case with the optimal stent brand for that particular off-label combination. As a consequence of an individualized stent brand selection approach we may potentially yield an outcome for the population which is superior to the outcome obtained by a general stent brand selection approach―even though one stent brand might have proven―at the average―to be generally the better stent brand (Figure 3).
Introduction of a new stent should be accompanied by clinical investigations with an intense characterization of both lesions and participating patients followed by recording of clinical events. From such a source the calculation of the HR-equation to use in the future may be extracted. If all new stents were characterized by an equa-
![]()
Figure 3. Cumulative proportions of lesions experiencing major adverse cardiac events during five years follow up. The lesions were grouped into one group that had received the optimal stent at the randomization time according to the individualized stent brand selection approach. The other group is the sirolimus(cypher)-eluting stent group representing the group treated in a generalized stent brand selection approach with the same stent brand to all lesions. Abscissa: days. Ordinate: percentage.
tion for individualized stent brand selection, then each individual with exactly those combinations of patient and lesion characteristics could be entered into the different equations, and eventually the stent brand with the lowest HR would be a fairly good choice.
6. Conclusion
There was no statistically significant difference in MACE rates between sirolimus and paclitaxel treated lesions. With increasing number of concomitantly appearing off-label characteristics we found a statistically significant stepwise increase in MACE rate from 16% to 32% in cases with 5 off-label characteristics. For each stent brand the HRs for MACE connected to 18 different off-label characteristics were estimated. If the clinically most important off-label characteristics are utilized to select the presumed better stent in each singular case then the populations MACE rate may be reduced by 10% - 18%. If introduction of all new stents was accompanied by HR for MACE for each off-label characteristic then the optimal stent brand to use in each case may be identified. This might increase stent performance and the clinical outcome for the population thereby would be optimized.
7. Study Limitation
The stents in the present study are no longer in use, and the exact HR for each off-label characteristic is now unimportant. Rather, the HR values serve to exemplify the impact of an individualized stent brand selection approach. It is therefore of no importance that the two stents are not in use anymore. Even a new study on the same matter, with present day stents would probably be outdated when the results of five years observation are ready. But present day stent performance may potentially be improved if the individualized stent brand selection approach were applied. The concept may therefore be important to investigate.
The MACE rate is not very lesion specific outcome measure. Multiple Cox regression with inclusion of 18 parameters requires a lot of events. The study sample should be much higher if the outcome parameter was focused to be lesion specific like a composite of proven re-stenosis and definite stent thrombosis. The present 2888 lesions observed for 5 years did not produce enough such events. The use of MACE is still justified because it merely serves to illustrate the impact of an individualized stent brand selection approach.
The Cox regression equations are drawn from the 2888 lesions, and are subsequently used to identify if a lesion in fact had been treated by an optimal or the worst stent brand. The correct approach would have been to use the equations in another sample of lesions, but this has not been possible because our lesion sample is the only sample with knowledge of 18 of-label characteristics. Being aware of this major drawback we still accept to commit such an error because it serves to illustrate the individualized stent selection principle. When comparing the MACE curve for lesions treated with sirolimus to the lesions treated with the presumed optimal stent brand we found 10% reduction in hazard for MACE among the latter group. When calculating the risk reduction we found an effect of 18%. Notably, the true effect among sirolimus- or paclitaxel-eluting stents remains unknown.
There is uncertainty of independence between lesions because some patients contributed with more than one lesion. The Cox regression analysis was consequently repeated in patients with only one lesion in order to assure that the HRs did not differ importantly from the ones deducted from the n = 2888 sample. If we had only utilized those patients the number of off-label characteristics had to be reduced due to insufficient number of events. As the HRs were only slightly changed we accepted to use the 2888 lesions―again simply because they merely serve to exemplify the individualized stent selection approach.
The correct proof of concept would necessitate a new randomized trial. Thus, Figure 3 may only be used to visualize the concept. The magnitude of the maximally achievable effect of an individualized stent brand selection approach remains to be proven.
Acknowledgements
The Danish Heart Registry (DHR) has contributed with essential detection of invasive cardiac procedures.
The SORT OUT II investigators: Niels Bligaard, Leif Thuesen, Henning Kelbæk, Per Thayssen, Jens Aarøe, Peter R. Hansen, Jens F. Lassen, Kari Saunamäki, Anders Junker, Jan Ravkilde, Ulrik Abildgaard, Hans H. Tilsted, Thomas Engstrøm, Jan S. Jensen, Hans E. Bøtker, Søren Galatius, Carsten T. Larsen, Steen D. Kristensen, Lars R. Krusell, Steen Z. Abildstrøm, Evald H Christiansen, Ghita Stephansen, R.N., John Godtfredsen, Søren Boesgaard, Jørgen L. Jeppesen, Anders M. Galløe.
The Independent End Points Committee: Jørgen L. Jeppesen, Søren Boesgaard and John Godtfredsen.
Clinicaltrials.gov: NCT00388934.
Conflicts of Interest
This work was supported by Boston Scientific and Cordis, a Johnson & Johnson company with unrestricted research grants but they had no role in the design and conduct of the study, the collection, management, analysis, or interpretation of the data, or the preparation, review, or approval of the manuscript.
NOTES

*Corresponding author.