A Potentiometric Evaluation of Stability Constants of Two-Step Overlapping Equilibria via a Bilogarithmic Hyperbolic Cosine Method ()
Received 7 February 2016; accepted 20 June 2016; published 23 June 2016

1. Introduction
The exact determination of the thermodynamic formation constants of many dibasic acids is complicated by the overlapping [1] - [4] of the successive ionization steps. A great many methods have been derived [5] - [7] for the potentiometric evaluation of formation constants of two-step simultaneous equilibria. Of them, methods based on the formation function [8] - [12] , ñ = f(pH), have been, undoubtedly, the most widely applied. The present paper describes a procedure for the study of stepwise equilibria in potentiometric titration, which is also based on Bjerrum’s function. Data (ñ, pH) are linearized according to a bilogarithmic mathematical model via a hyperbolic cosine method relationship. The treatment of the (ñ, pH) data by the procedure derived in this paper does not require that the ionic strength is maintained constant by addition of inert salt. This paper forms part of an investigation [9] [13] into the uses of bilogarithmic methods and hyperbolic functions in parameter estimation. Methods based on the application of spectrophotometric measurements have been the subject [14] - [16] of recent studies.
2. Theory
For a diprotic acid H2R, the average proton number [7] [9] [10] [17] [18] (the average number of proton bound per R) is given by
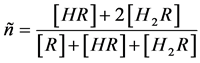 (1)
(1)
where charges have been omitted for convenience. The stepwise thermodynamic formation constants of the acid is defined by
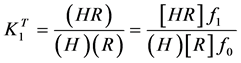 (2)
(2)
 (3)
(3)
where parenthesis indicate activities and braces concentrations; f2, f1 and f0 being the activity coefficients of the species H2R, HR and R, respectively.
By combining Equations ((1)-(3)) we get
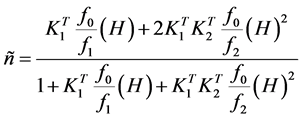 . (4)
. (4)
On rearrangement Equation (4), we obtain
 . (5)
. (5)
Two different situations will be considered in that follows depending whether the proton number values were lower or higher than the unity.
2.1. Procedure for Average Number Values Lower Than the Unity
By dividing Equation (5) by (H)3/2, a further rearrangement leads to
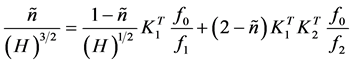 . (6)
. (6)
By multiplying and dividing the right hand of Equation (6) by
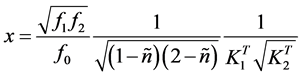 (7)
(7)
we get
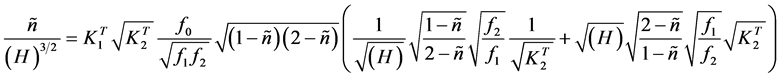 . (8)
. (8)
Making
 (9)
(9)
and taking into account that
 . (10)
. (10)
Equation (8) may be converted into
 (11)
(11)
where pH = −log(H). By taking logarithmic on both sides of Equation (11), on rearranging we finally get
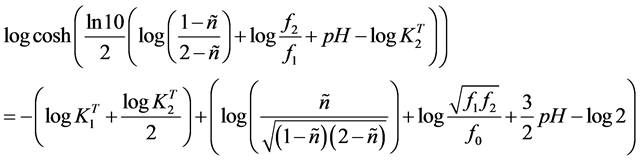 . (12)
. (12)
Thus, a representation of the left term of Equation (12) against the term into brackets of the right hand should give a straight line (Y = a0 + a1X), obtained by linear regression [19] - [22] , whose slope is the unity and the intercept with the X-axis is equal to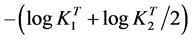 , from which the
, from which the  may be estimated as
may be estimated as
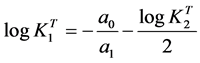 . (13)
. (13)
The application of Equations ((12) and (13)) requires, however, the previous knowledge of![]() . Different values of
. Different values of ![]() may be assumed and the entire procedure then applied. The best value of
may be assumed and the entire procedure then applied. The best value of ![]() may be taken as that satisfies an optimization criterion, e.g. that minimizes the mean quadratic error (MQE) in ñ measurements
may be taken as that satisfies an optimization criterion, e.g. that minimizes the mean quadratic error (MQE) in ñ measurements
![]() (14)
(14)
where N is the number of data pairs, and ñ is calculated from Equation (4) once both logK values are known, This task is easily carried out with the aid of an Excel spreadsheet.
In those cases in which the ionic strength is held constant by addition of an inert salt, e.g. potassium chloride or potassium nitrate 0.1 M, Equation (12) is converted into
![]() (15)
(15)
where ![]() and
and ![]() are mixed or Bronsted constants, whose dependence on ionic strength can be expressed by
are mixed or Bronsted constants, whose dependence on ionic strength can be expressed by
![]() (16)
(16)
![]() , (17)
, (17)
![]() and
and ![]() are the stoicheiometric constants and fH the activity factor of hydrogen ion. Note that the ñ values when ionic strength is held constant are given by
are the stoicheiometric constants and fH the activity factor of hydrogen ion. Note that the ñ values when ionic strength is held constant are given by
![]() . (18)
. (18)
2.2. Procedure for Average Number Values Greater Than Unity
In these situations, by dividing Equation (5) by (H)1/2, on rearrangement we get
![]() . (19)
. (19)
By multiplying through
![]() . (20)
. (20)
Equation (19) is converted into
![]() . (21)
. (21)
Taking into account the definition of hyperbolic cosine first and taking decadic logarithms on both sides of the resulting equation then, a posterior rearrangement leads to
![]() . (22)
. (22)
When the left term of Equation (22) is plotted against the term into brackets of the right hand, a straight line (Y = a0 + a1X) of unity slope should be obtained, from which the value of ![]() may be estimated as
may be estimated as
![]() . (23)
. (23)
Nevertheless, before Equation (22) can be applied, ![]() must be known. A procedure analogous to that suggested in the previous section may be followed in order to circumvent this difficulty.
must be known. A procedure analogous to that suggested in the previous section may be followed in order to circumvent this difficulty.
If the ionic strength is maintained constant during the titration then
![]() . (24)
. (24)
The basis of this discussion has been protonation reactions, but the same principles apply for metal complexation reactions M + L = ML and ML + L = ML2
![]() (25)
(25)
![]() (26)
(26)
being the formation function or Bjerrum index in this case
![]() . (27)
. (27)
2.3. Ionic Strength Expression
Taking into account that V0 millilitres of the diprotic acid H2R at a concentration CA moles/liter, haven been titrated with a volume V of titrant, e.g. a strong monoacid base BOH, of concentration CB moles/liter, the computation of ionic strength may be made assuming the Speakman [23] expression corrected by the volume, as a first approximation. Then, if CBV < CAV0
![]() . (28)
. (28)
In those cases in which CBV > CAV0 we get
![]() . (29)
. (29)
The Debye-Hückel equation [24] - [26] (or other more sophisticated one) may be employed for the ionic activity coefficients and unity assumed for the activity of the uncharged molecule H2R
![]() (30)
(30)
where A and B are constants of the Debye-Hückel theory, and ä is the so-called ion-size parameter, or some extended form of the empirical Debye-Hückel equation as the Davies equation [27] . The activity coefficient may be evaluated if required by standard iteration to constant fi.
2.4. Error Analysis
In those cases in which ñ < 1, the straight line intersect the X-axis at the point
![]() (31)
(31)
from which we may evaluate the value of ![]() once the value of
once the value of ![]() is known.
is known.
By applying the law of random error propagation [28] we get
![]() . (32)
. (32)
Taking into account [19] - [22] [28] the expressions for![]() ,
, ![]() and
and ![]() are given by
are given by
![]() (33)
(33)
where
![]() . (34)
. (34)
An estimate of the uncertainty of these calculations is given by
![]() (35)
(35)
In those cases in which ñ > 1 then
![]() (36)
(36)
and the application of the random error propagation law gives in this case
![]() . (37)
. (37)
2.5. Choice of Starting Values
Two principal difficulties should be self-evident. Primarily the present analysis requires a prior estimate of the individual stability constants. On this respect, preliminary values of ![]() and
and ![]() may be evaluated [9] [29] from Equations ((35) and (36)) by considering three well defined points on the titration curve at ñ = 0.5, 1.0, and 1.5
may be evaluated [9] [29] from Equations ((35) and (36)) by considering three well defined points on the titration curve at ñ = 0.5, 1.0, and 1.5
![]() (38)
(38)
![]() (39)
(39)
where
![]() . (40)
. (40)
Expressions (35) and (36) are only approximate because of the influence of varying ionic strength. In addition, it is always disadvantageous to calculate stability constant from a minimum amount of experimental data. As a matter of fact, however, even the pH values of ñ = 0.5 and ñ = 1.5 may be taken as starting point for ![]() and
and ![]() values, respectively.
values, respectively.
3. Applications
In order to check the usefulness of the method it has been applied to a variety of systems previously described in the literature. Systems chosen for study were representative of the most difficult experimental situation encountered in practice. All have log K values similar in magnitude thus being very suitable for the purpose of this work. Experimental details and [pH,V] and [pL,ñ] data employed are given in that follows:
I. Succinic acid [24] : CR = 0.005 M; V0 = 100 mL, CB = 0.1 M (KOH); T = 25˚. Data [V, pH]: [1.00, 3.677; 1.25, 3.767; 1.50, 3.853; 1.75, 3932; 2.00, 4.009; 2.25, 4.081; 2.50, 4.153; 2.75, 4.223; 3.00, 4.291; 3.25, 4.361; 3.75, 4.498, 4.00, 4.569; 6.00, 5.135; 6.25, 5.204; 6.50, 5.273; 6.75, 5.342; 7.00, 5.412; 7.25, 5.480; 7.50, 5.554; 7.75, 5.629; 8.00, 5.208; 8.25, 5.789; 8.50; 5.881; 8.75, 5.981; 9.00, 6.099].
II. Cu(II)-Glicine system [18] at T = 25˚C. Data [pL, ñ]: [8.667, 0.250; 8.607, 0.270; 8.549, 0.296; 8.492, 0.326; 8.423, 0.351; 8.358, 0.385; 8.294, 0.426; 8.221, 0.463; 8.150, 0.511; 8.076, 0.564; 7.993, 0.620; 7.902, 0.681; 7.803, 0.749; 7.715, 0.807; 7.630, 0.872; 7.215, 1.169; 7.084, 1.251; 6.975, 1.139; 6.838, 1.425; 6.708, 1.515; 6.565, 1.606; 6.380, 1.697; 6.192, 1.788; 5.886, 1.880].
III. Silver(I)-4-aminobutan-1-ol [30] [31] at T = 20˚C and I = 0.5. Data [pL, ñ]: [4.198, 0.261; 4.121, 0.327; 4.058, 0.392; 4.000, 0.458; 3.950, 0.523; 3.906, 0.589; 3.861, 0.654; 3.818, 0.719; 3.780, 0.785; 3.740, 0.850; 3.700, 0.915; 3.59, 1.110; 3.549, 1.110; 3.516, 1.238; 3.477, 1.303; 3.389, 1.429; 3.292, 1.553; 3.173, 1.671; 3.023, 1.779; 2.824, 1.862].
Figure 1 shows the application of the bilogarithmic hyperbolic cosine method (BHCM) to the succinic acid system. The residuals obtained were [− + + + − + − − − + − + +] and [+ + − + − − − + − + + + −] for ñ < 1 and ñ > 1, respectively, then show no special pattern. A well defined unity slope, 1.0009 ± 0.0043 and 0.9985 ± 0.0024, respectively, was obtained in both cases. The results obtained by means of the BHMC method are in good agreement with the values obtained by Albert and Serjeant [25] by applying a computerized FORTRAN method.
The ideal methodology devised for H2R/HR/R systems may be applied to simultaneous complex systems ML2/ML/M. In this case the data available are (pL, ñ). Figure 2 and Figure 3 show the application of the BHMC method to the Cu(II)-glycine and Ag(I)-4-aminobutan-1-ol systems, respectively. Irving and Rossotti (18) obtained for the Cu(II)-glycine system (Table 1) values of log K1 of 8.12 to 8.16 and log K2 of 6.73 to 6.78. The results obtained in this paper are [8.177 - 8.143] for log K1, and [6.772 to 6.645] for log K2. The values obtained for ñ < 1 and ñ > 1 differ in 0.034 and 0.127 log units, for log K1 and log K2, respectively. The slopes of our method in both cases are close to 1 (0.9787 ± 0.0271 for ñ < 1 and 0.9878 ± 0.0134 for ñ > 1).
![]()
Figure 1. Top left: Mean quadratic error (MQE) as a function of log K2 assumed (ñ < 1). Top right: Bilogarithmic plot (ñ < 1) for the succinic acid system. Bottom left: Mean quadratic error (MQE) as a function of log K1 assumed (ñ > 1). Bottom right: Bilogarithmic plot (ñ > 1) for the succinic acid system.
A well defined slope (1.0073 ± 0.0136) was obtained for the system Ag(I)-4-aminobutan-1-ol (ñ > 1) and values of log K1 and log K2 of 3.416 ± 0.002 and 3.896 (assumed), respectively. Lansbury et al. [30] and Unwin et al. [31] obtained values of 3.41 and 3.89, respectively, using computerized methods based on the use of weighted least squares, and response surfaces, respectively. The results obtained by applying the BHMC method proposed in this paper coincide with those provided by these authors. The Ag(I)-4-aminobutan-ol system, however, departs from a behaviour model at ñ > 1 values.
4. Conclusion
A major goal of scientific experimentation is the discovery of relationships [32] among variables. The evaluation of stability constant by linearized plots on this respect seems to be more prevalent, probably owing to the transparency [33] of the methods used. Note that non-linear least squares are not always problem-free. Occasionally, problems arise [34] because of the choice of the data, initial estimates, convergence or multiple local minima, and all-typical of non-linear regression. A main advantage of the bilogarithmic method devised in this paper is that a theoretical slope of unity should be obtained this proving directly the correctness of the assumed equilibria. Significant deviation from this behaviour is indicative of more complicated phenomena. It is interesting to note
![]()
Figure 2. Top: Graphical representation for the ñ versus pL data. The curve in the figure is calculated with logK1 and logK2 given in Table 1 (bilogarithmic method). Bottom left and right: logarithmic plots.
![]()
Figure 3. Top: Graphical representation for the ñ versus pL data. Bottom left and right: Bilogarithmic plots.
![]()
Table 1. Comparison of results obtained by different methods in the evaluation of formation constants.
that by applying other least-squares procedures, it is not possible to determine whether a given pH against fraction titrated curve is characterized only by the assumed reactions. In this respect, when the independent and dependent variables are varied over a number of orders of magnitudes, the points tend usually [17] to be bunched together. However, an additional advantage of the bilogarithmic method reported here provides a closed scale representation of y and x, unlike other plots. The bilogarithmic hyperbolic tool devised here, for all reasons indicated above, constitutes an appropriate and useful mathematical model for the potentiometric study of simultaneous equilibria.
NOTES
![]()
*Corresponding author.