Rapid and Continuous Extraction of Methyl Red from Wastewater Using Counter Current Chromatography ()
Received 4 March 2016; accepted 10 June 2016; published 13 June 2016

1. Introduction
It is estimated that more than 100,000 commercially available dyes with over 7 × 105 tons of dyestuff are produced annually [1] [2] . As usual dyes are used in textile, paper, plastic, leather and many other industries. So, dyes releasing from these industries cause eco-toxic hazard and may eventually affect human through food chain. In addition, some dyes are either toxic or mutagenic and carcinogenic [3] [4] . It is reported that, approximately one million kilograms per year of a dye are discharged into water streams just by the textile industry [5] [6] . The most widely used industrial dyes are azo dyes, which constitute 60% - 70% of all produced dyestuffs [7] - [9] .
The extensive use of dyes could spread carcinogenic, mutagenic, allergenic and environmental pollution [10] . It is, therefore, essential to remove the dye from wastewater or treat it in such a way so as to minimize the damage to the environment [11] [12] . When a dye is stable under all conditions (exposure to sunlight, water, soap, soil etc.), it becomes more difficult to treat it in wastewater [13] . These hazardous contaminants must be removed from waste effluents prior to discharge. Azo dyes are exclusively organic compounds consisting of a diazotized amine coupled to an amine or a phenol and contain one or more azo (-N=N-) linkages [14] [15] .
Methyl red is a kind of anionic azo dye [16] and causes irritation of the eyes, skin and digestive tract if inhaled/swallowed [17] [18] .
Coagulation, flocculation, oxidation or ozonation, membrane separation and activated carbon adsorption are known as the conventional methods for treating wastewater containing dyes [19] . Nowadays much attention has been given to more effective and rapid chemical separation techniques such as solvent extraction which is used for the purification, enrichment and analysis of various compounds in mixtures. This technique is based on the principle that a dye can distribute itself in a certain ratio between immiscible solvents. Therefore the selection of both a diluent and an extractant determines the equilibrium for a given system, so, efficiency of extraction process depends on its mass transfer rate [20] [21] . Muthuraman reported the extraction and recovery of methylene blue from industrial wastewater by LLE, using benzoic acid as the extractant [22] . Under optimized conditions, 98% of the dye was extracted from aqueous solutions and the extracted dye in the organic phase was back extracted into sulphuric acid solutions. Similarly, methyl red dye was extracted and recovered from industrial wastewater by LLE technique. Extraction efficiency varied from 90% to 97% at the aqueous to organic phase and the loaded dye was back extracted into sodium hydroxide solutions [23] . Recent literature is an effective and attractive process for the decontamination of the dye-containing effluents and many researchers have adopted the adsorption approaches [24] [25] . The other methods such as coagulation and flocculation [26] - [28] and other materials such as activated carbon are also used for the removal of methyl red from simulated wastewater [29] - [31] . In the present work the extraction of anionic dyes namely methyl red from wastewater solutions was carried out by Low Speed Counter Current Chromatography (LSCCC). This is a modern technique for the separation and purification of the samples [32] - [36] . CCC is a form of support-free chromatographic system. It is simple operation and less time-consuming technique. It is essentially suitable for separation and purification of high polar compounds. Using CCC in preparative and continuous separation is steadily growing [37] [38] .
In our best knowledge, no paper has been reported on the use of LSCCC for the extraction of dyes from wastewater. The fundamental parameters such as pH, effect of time, stripping agent, initial dye concentration and effect of diluents on dye extraction efficiency were studied. Using LSCCC a high efficient recovery for methyl red was obtained. The method was compared with data obtained at the same conditions with liquid-liquid extraction (LLE) method.
2. Experimental
2.1. Materials and Apparatus
All chemicals used in this study were of analytical grade reagents. Methyl red, xylene, sulphuric acid, sodium hydroxide were obtained from Merck. UV-visible spectrophotometer (Lambda 25) was used to measure the absorbance of dyes to establish its λ max and its concentration. The pH was measured by a pH meter (model 320 Mettler Toledo Instruments). For agitation of solutions a mechanical stirrer (IKD_Ks50) was used. Xylene was used as an extractant and distilled water was used for preparation of the dye solution. The pH was adjusted by sulphuric acid.
2.2. LSCCC Instrument
The LSCCC was constructed in our lab. The column is made from a long piece of polytetrafluoroethylene (PTFE) tubing by winding it around a cylindrical holder hub to make multiple layers of coil. The column is mounted in a centrifuge rotor, which rotates around the central axis at a known angular velocity (ω), as in an ordinary centrifuge system.
The length of the column was a 1.5 meter tube with a 6 mm diameter and the solutions were injected using an HPLC pump (Water, model 515, M Ilford MA). The stationary and mobile phases were pumped into multilayer column at a flow rate of 1 ml/min in the head to tail direction.
The stationary upper phase [(xylene) (20 mL)] loaded into the column by syringe. Then a volume of the prepared aqueous dye solution (20 mL) introduced into the column and head and tail of column was interconnected. The column is rotated for a known time at a very low speed about 15 to 60 rpm. The effluent mixture was transferred into a separating funnel and was allowed to settle both layers. Aqueous layer (lower phase) was taken for absorbance measurement. Chemical structure of the methyl red (Figure 1) contains one (-N=N-) linkage and the wavelength of maximum absorption (λ max) for methyl red was 520 nm. Characteristics of methyl red are described in Table 1. The distribution ratio (D) and percentage of extraction (E) were calculated by the Equations (1) and (2):
 (1)
(1)
 (2)
(2)
where 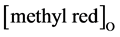 is the methyl red concentration in organic phase (mol/L), [methyl red]A is the methyl red concentration in aqueous phase.
is the methyl red concentration in organic phase (mol/L), [methyl red]A is the methyl red concentration in aqueous phase. 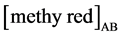 and
and 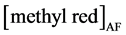 are the initial and final concentration of methyl red in aqueous phase before and after extraction, respectively.
are the initial and final concentration of methyl red in aqueous phase before and after extraction, respectively.
To study the effect of the stripping agent, 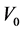 mL extractant and
mL extractant and 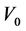 mL sodium hydroxide solution as stripping agent added together into the LSCCC column and rotated at 60 rpm. The content was then transferred into a separating funnel. The aqueous phase at the bottom of the separating funnel was taken for dye concentration measurements.
mL sodium hydroxide solution as stripping agent added together into the LSCCC column and rotated at 60 rpm. The content was then transferred into a separating funnel. The aqueous phase at the bottom of the separating funnel was taken for dye concentration measurements.
2.3. Liquidé-Liquide Extraction Procédure
The organic solvent (xylene) used for extraction was added to the known volume prepared aqueous dye solution in a glass-stoppered bottle and the glass-stoppered bottle was shaken in a shaker at 60 rpm. The mixture was then transferred into a separation funnel. Then the extracted dye was taken for absorbance measurement at 520 nm.
3. Results and Discussion
3.1. LSCCC Mechanism
The mechanism of the CCC separation is based on the Archimedean screw force which mixes two solvent and
![]()
Figure 1. Chemical structures of anionic methyl red.
![]()
Table 1. Characteristics of methyl red.
yields excellent partition efficiency for a large scale sample. The CCC technique also faces to some problems such as band broadening due to longitudinal phase mixing and needs high column pressure. In order to cope with these problems, we have developed a short column, low speed CCC (LSCCC), which causes rapid circular motion of two solvent phases in cylindrical compartment.
Figure 2 shows the LSCCC as a prototype, has been constructed in chromatography lab-IROS. The holder, rotates on its own axis while revolving around the central axis.
When the filled coiled is held horizontally and slowly rotated on its own axis, both immiscible solvents (heavier and lighter) moves toward one end of the coil. The slow rotation could establish a hydrodynamic equilibrium between the two solvents. In this condition and under hydrodynamic equilibrium, one phase can move at the head, while the other phase retaining in the coil, causes solutes to be subjected to an efficient partition process and separation according to its partition coefficient between two liquids. In two solvent system the aqueous phase was the lower one, and extraction solution was pumped into the LSCCC column eluted by the mobile phase. After completion of the first cycle, the second one could be carried out by the same procedure. Obtained data indicated that both spiral effect and Archimedean screw force contribute to a high partition efficiency.
For this research work five important factors have been studied to evaluate the performance of the extraction method. These factors are stripping agent, effect of pH, effect of initial dye concentration, time and dilution effects which are discussed in following sections.
3.2. Stripping Studies
The efficiency of separation of methyl red from aqueous phase mainly depend on a suitable solvent system. To obtain optimal two-phase solvent system, different solvents were used as mobile phases. The solvents examined were benzene, toluene, xylene and hexane. Among them the extraction efficiency of the benzene, toluene and xylene were acceptable while hexane was unable to extract dye from aqueous solution. Figure 3 shows that in all cases the efficiency of the LSCCC is more than LLE technique. Based on this experiment, and because of its less toxic effect compared to benzene and toluene and it’s more effective than hexane to study the extraction of methyl red from aqueous solution, xylene was used as an extractant by a new method called Low Speed CCC. An optimal solvent system composed of xylene-water (1:1, v/v) was selected for LSCCC separation of ionic methyl red from the industrial wastewater. Various factors such as the effects of pH, initial dye concentration and equilibration time on extraction efficiency of methyl red from aqueous solution were performed.
![]()
Figure 2. An illustration of LSCCC column.
![]()
Figure 3. The effect of solvent on extraction of methyl red using LSCCC and LLE.
The extraction phase ratio studies, shows that maximum efficiency could be achieved when phase ratio is 1:1 to 3:1 (aqueous phase to organic phase). When the phase ratio increase to more than 4:1 and 5:1 the extraction efficiency dropped from 98 % to just 91 and 83 percent respectively.
The organic solvent has been used after the first extraction and again interred into the LSCCC column to reextract methyl red from aqueous solution. Table 2 shows that the efficiency of the extraction was constant up to 4 cycles and then decreased smoothly.
3.3. Effect of pH in the Aqueous Phase
The effects of initial pH on extraction percentages of dyes were researched over a range of pH values from 1 to 7.5 ± 0.1 at 25˚C. The results show that extraction efficiency decreased with increasing pH. The maximum extraction of dye was noticed at pH 2 - 4.5 as shown in Figure 4. At low pH and pH > 4.5, the extraction efficiency decreased sharply. At the low pH, H+ ion concentration was increased in the solution and combines with anionic methyl red, so improves its solubility in xylene. At high pH, the methyl red is in its unionized form, so it shows hydrophobic interaction with the stationary phase. It was shown that a sudden change in pH value causes to extreme changes in the retentions of the methyl red. To study other factors, the pH maintain at 2.5 ± 0.1 at 25˚C and the aqueous phase concentration was maintained at 2 × 10−4 mol/L.
3.4. The Effect of Initial Dye Concentration in Aqueous Phase
The effect of initial dye concentration in aqueous phase on the extraction efficiency of the methyl red were investigated in the range of 1 × 10−4 to 8 × 10−4 mol/L.
Extraction efficiency was not significantly changed in these range. But generally the results show that extraction efficiency first increased with increase the concentration up to 2 × 10−4 mol/L and more than this amount, efficiency decreased sharply (Figure 5). It seems that at higher dye concentration, xylene could not completely extract the dye from the aqueous phase.
3.5. Effect of Equilibration Time
The extraction of methyl red using LSCCC and LLE were studied for the period of 1 - 15 min. As shown in Figure 6, after 5 min, the efficiency of the extraction was not changed significantly. Hence, a fast five min extraction time was chosen for further studies.
3.6. Removal of Methyl Red from Real Wastewater
The real wastewater sample obtained from Baft Azadi Textile Co, Tehran. I. R. Iran. The sample contain various
![]()
Figure 4. Effect of pH on extraction efficiency of the methyl red. Experimental conditions: solvent system, xylene-water (1:1/v/v), volumeof aqueous phase = 20 mL at different pHs, volume of organic phase = 20 mL, dye concentration 0. 2 mmol/L, equilibration time = 5 min.
![]()
Figure 5. Effect of dye concentration on extraction efficiency of the methyl red. Experimental conditions: solvent system, xylene-water (1:1/v/v). Volume of aqueous phase = 20 mL at pH = 2.5 ± 0.1, volume of organic phase = 20 mL, dye concentration 0.2 mmol/L to 0.8 mmol/L, equilibration time = 5 min.
![]()
Figure 6. Effect of time in extraction efficiency of the methyl red. Experimental conditions: solvent system, xylene-water (1:1/v/v) volume of aqueous phase = 20 mL at pH = 2.5 ± 0.1, volume of organic phase = 20 mL, dye concentration 0.2 mmol/L, equilibration time = 1 - 15 min.
![]()
Table 2. The percentage of re-extraction of methyl red by reused solvent.
types of salts and was alkaline in nature. Optimized conditions were applied and the results of 5 replicate test show that over 98.4% of the dye could be extracted into organic phase using LSCCC technique. Comparing to synthesis aqueous phase it was concluded that no significant differences between real and synthesis sample has been observed.
4. Conclusions
In this study, the aim was extraction of methyl red from wastewater. Methyl red was extracted from aqueous solution by LSCCC. The selected solvent system was xylene-water (1:1 v/v) and xylene (extractant) was used as organic phase. Five various factors were evaluated and it was found that the extraction efficiency was about 99.7% in just 5 min in the optimal conditions. Maximum extraction was observed in a pH range of 2 to 4.5.
The importance of this report is not just to propose a new method for extraction of dyes, but also to demonstrate a high efficiency technique which could be used for preparative and large-scale removal of dyes from aqueous phase.
NOTES
![]()
*Corresponding author.