Synthesis and Characterization of Triply Doped Nano-Composite Alumina-Phospho- Silicates SiO2-P2O5-Al2O3 with Er3+, Sm3+ and Yb3+ Ions Prepared by Sol Gel Technique in Two Different Forms Thin Film and Monolith ()
Received 17 May 2015; accepted 18 January 2016; published 21 January 2016

1. Introduction
With introduction of internet super highway and increasing demand for broadband data transmission, there is an increasing demand for performance materials with high reliability and low cost small form factor components to meet the needs of miniaturised devices. Such components (including couplers, splitters, tunable lasers, optical amplifiers, etc.) require high grade materials and industrial low cost effective processes. Indeed, sol-gel process appeared to us as one of the best processes to consider. Interaction between SiO2 and P2O5 derivatives could serve as a model for the precursor coating in the integrated optics technology. Silicon oxide resulting from Si device could serve as interface for a better adherence of the film.
Incorporating rare earth ions in the host matrix also allow controlling its refractive index as well as its optical activity, absorption etc. However, a limitation for such optical devices is the precipitation of rare earth oxides or insoluble phospho-silicates. Therefore, it is worth to investigate the sol-gel preparation process of the prepared samples that determine the highest concentration of rare earth allowed without running into uncontrolled crystallization and clustering problems. It is also of importance to underline the chemical durability of the materials as most of P2O5 based compound suffers from its hygroscopic character [1] -[5] .
In the present work, a simple sol-gel procedure was successfully used for the preparation of alumina-phospho- silicate as monolith and thin film forms prepared from the same precursor materials, using the tetra-ethoxysilane and triethyl-phosphate precursors. Silica-phosphate glasses activated by Sm3+, Er3+ and Yb3+ ions were prepared by sol-gel route, using monolith and spin-coating methods. The structure of the prepared samples will be evaluated by using XRD. The effect of co-incorporating these two forms of materials with Sm3+, Er3+ and Yb3+ ions on the structure and optical properties will be discussed.
2. Experimental
The investigated nano-composite Alumina-Phospho-Silicates (SiO2-P2O5-Al2O3) pure and Triply doped with three different rare earth ions (REIs) (Er3+:Yb3+:Sm3+) have been prepared by sol-gel technique in monolith and thin film forms with sintering at different temperatures ranging from 200 up to 900˚C. The starting materials used in this study are tetraesoxisilane and triethyl phosphate for SiO2 and P2O5 precursors, respectively. The other trivalent oxides are incorporated using nitrate solutions as shown in the flow chart of samples preparation given in Figure 1.
As shown in Figure 1, the monolithic and thin film forms were obtained by hydrolysis and poly-condensation of tetra-ethoxysilane (CH3CH2OH)4Si (TEOS, 99.999%, Sigma-Aldrich) and Triethyphosphate (C2 H5O)3P(O) reacted in ethanol solution under vigorous stirring with distilled H2O containing HCl used as a catalyst. Then the
![]()
Figure 1. Flow chart for the preparation of Alumina-Phospho- Silicates gels doped with (Er3+, Er3+: Yb3+& Er3+: Yb3+: Sm3+) preparation in monolithic and thin film forms.
Er3+, Yb3+ and Sm3+ ions were introduced in the process, by mixing with rare earth nitrate solutions with molar ratios of 1.2 and 1.8 mol% of Er2O3 and Yb2O3 and Sm2O3 of 0.7, 1.1 and 1.3 mol%, and 3 mol% of Al2O3, were added, respectively. In order to increase the solubility of rare earth in the glass matrix due to the valence match between the rare-earth dopant (RE3+) and the substituted cation (Al3+). The resulting homogeneous solutions were used to prepare both monolithic and thin film materials using the process indicated hereafter in reference [4] :
1) Preparation of monolith samples: Solutions were filled in a mold and aged for one week at room temperature and then dried in a drying oven type GFL 71.5 at about 60˚C for about 21 days until no further shrinkage appears. Samples were found to be clear, transparent and cracks free. Densification of gel was obtained by annealing in air for three hours at temperature ranging from 60 up to 900˚C in a muffle furnace with heating rate 1.5˚C/min as reported previously by our team work [6] -[10] .
2) Thin film preparation: The remaining part of the resultant homogeneous solutions of monolith materials were used in the preparation of thin film. In this case the solutions were aged for one day at room temperature before to be dispersed on the glass and/or silica substrate, with a spun of 3500 rev./min for 30 seconds in a clean room. At least two successive coatings were required to provide suitable effective film thickness. After finishing the coating process, the films dried for 30 min and then sintered at temperature ranging from 100˚C up to 700˚C [8] .
Different typical examples of X-ray diffraction (XRD) patterns recorded for some monolith and thin film samples are given in Figures 2-5. Crystallite sizes G were determined using the Scherer’s equation
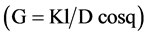 (1)
(1)
with (K) Scherer constant (0.9), (l) X-ray wavelength, at D (radian) = the full width at half maximum (FWHM) of the diffraction peak and q = diffraction angle.
Microstructure and morphology for the pure Alumina-Silica-Phosphate were characterized using “JEOL transmission electron microscope” (Model: Jeol 1230 magnification up to 600 kx, resolution down to 0.2 nm, accelerating voltage 100 kV, can reach 120 kV through steps).
For photoluminescence (PL) measurement the samples were excited using the 514 nm line of a Spectra Physics 2017 Argon laser. A fiber optic probe coupled to a Dilor Super head, equipped with a suitable notch filter was employed.
The chemical composition given in Table 1 in terms of the starting mixture of the precursors used according to the chart given in Figure 1.
3. Results and Discussion
XRD Analysis
As expected the (as prepared) samples monolith and thin films both sintered at 900˚C for three hours, results in a better crystallization of the analyzed XRD patterns. However even after a sintering of 3 hours at 900˚C, a slight remain of amorphous phase is still observable on XRD patterns, as shown in Figure 2. Indeed the major constituents of all prepared monolith and thin films in the present study are SiO2 and P2O5. Therefore it is normal to expect the resulting crystalline phases to belong to the binary system SiO2-P2O5 or close to this line. All XRD patterns in Figure 2 tend to confirm that the crystalline phases, after sintering at 900˚C at constant Er3+ and Yb3+
![]()
Table 1. Equivalent oxide (mol %) of the starting mixture of the investigated materials.
![]()
Figure 2. XRD patterns of Alumina-Phospho-Silicates mono- lith samples triply doped with Er3+, Sm3+ and Yb3+ ions; SPAE1.2Y1.8 with different mol% of Sm2O3 equal to 0.7 (a), 1.1 (b) and 1.3 (c), as sintered for three hours at 900˚C.
![]()
Figure 3. (a) and (b) XRD patterns of Alumina-Phospho- Silicates thin film triply doped with Er3+, Sm3+ and Yb3+ ions; SiO2: 11 P2O5: 3 Al2O3: 1.2 Er2O3: 1.8 Yb2O3: 1.3 Sm2O3, as sintered for three hours at 900˚C.
![]()
Figure 4. TEM images of monolithic samples SPAE1.2Y1.8 doped with 0.7 (a) and 1.3 mol% Sm2O3 (b), as sintered for three hours at 900˚C.
ions concentration and different concentrations of Sm3+ ions are correspond to SiP2O7, Si5P6O25 and Si3(PO4)4, JCPDS [71-2073], [81-1593] and [49-0206], respectively. It is clearly seen that the intensity of the peaks increase by increasing the Sm3+ ions concentrations. Rare earth oxides and related compounds are in so small
![]()
Figure 5. The photoluminescence (PL) emission spectra of thin film SPAE1.2Y1.8S0.7, sintered for three hours at 500˚C.
quantities that they might not appeared with their corresponding X-ray diffraction patterns. The general behavior observed from the values of the crystallite size and the intensity increasing of the peaks by increasing the Sm3+ ions from 0.7 up to 1.3 mol% is its increase trend by doping with 1.3 mol% of Sm3+ ions, this may be due to the co-doped with Al3+ ions, which enhance the solubility of samarium inside the host matrix.
Figure 3 shows the XRD pattern of the thin film SPAE1.2Y1.8S1.3 sintered at 900˚C for three hours. Nearly the same phases obtained from XRD patterns of monolith samples are clearly appeared in thin film sample, but the peaks have lower intensity than monolith samples due to the low thickness of film.
The crystallite size of SPAE1.2Y1.8S1.3 sintered at 900˚C for three hours was of the order of 44 nm for monolith sample. By decreasing the Sm3+ ion concentrations the crystalline size was decreased [5] [11] [12] . It is also seems that the thin film SPAE1.2Y1.8S1.3 sample sintered at 900˚C, gave smaller crystalline size than monolith sample giving the following value 31 nm. The obtained XRD data are confirmed by using the Win-fit program.
The transmission electron microscopy TEM was used to confirm and complement the results obtained from XRD, as shown in Figure 4(a) and Figure 4(b) of monolith SPAE1.2Y1.8S0.07 (a) and SPAE1.2Y1.8S1.3 (b) both sintered at 900˚C. The patterns indicated the presence of the practically spherical with some agglomeration. The grain size was determined by averaging over the total number of grains in the TEM (Jeol1230) micrograph. Based on this method, the average crystallite size calculated from TEM was very close to the obtained from XRD for the same sample SPAE1.2Y1.8S1.3 and was found to be equal to about 36 nm.
Figure 5 shows the photoluminescence (PL) emission spectra under Argon laser excitation at wavelength (488 nm) of thin film SPAE1.2Y1.8S0.7, sintered for three hours at 500˚C. It is expected here that all the energy levels of Er3+:Yb3+ system will be reconstructed when doped with Sm3+ ions in Alumina-Phospho-Silicates system. It is well known that the absorption cross-section of Er3+ and Sm3+ ions are small, for that Yb3+ ions was used as a sensitizer to increase the Er3+ and Sm3+ ions PL emission and offering good spectral overlap with Er3+ and Sm3+ transition, thus allowing efficient Yb3+-Er3+-Sm3+ energy transfer with subsequent Er3+ emission. It has been well addressed that the green emissions at 534 nm have been attributed to the intra-4F-transitions of Er3+ and Sm3+ ions and were assigned for both rare earth elements to the (2H11/2―4I15/2) and 4G5/2―6H5/2, respectively. The group red emission is attributed to another 4F-transition of Er3+ and Sm3+ ions assigned to (4F9/2―4I15/2) for Er3+ ions. While it was assigned to 4G5/2―6H7/2 at 604 nm, 4G5/2―6H9/2 at wavelength 636, 644 and 652 nm, 4F3/2―6H11/2 at wavelength 685 nm, 4G5/2―6H11/2, for 702 and 715 nm, 731, and 4F3/2―6H13/2 for 795 and 810 nm in region between 600 and 800 nm for Sm3+ ions [12] -[21] .
The red emission is much stronger than green emissions, which may be due to effect of doping the prepared samples with Yb3+ ions. The introduction of Yb3+ ions in silica-phosphate host brings about great changes for the photoluminescence properties of Er3+ and Sm3+ ions and a moderate green to red light can be seen in thin film sample under the same excitation. Where the multiplicity of RE sites in the host matrix is known to enhance the inhomogeneous broadening of the emission and absorption lines and a red shift was observed for the emission lines.
4. Conclusion
Nano-composite Alumina-Phospho-Silicates doped with 3 different RE3+ erbium, ytterbium and samarium ions: (SiO2:11P2O5:3Al2O3:1.2Er:1.8Yb:(1.3)Sm), were successfully prepared by using a modified sol-gel technique, in two different forms monolith and thin film. The structure of the prepared samples was evaluated by using XRD, which revealed that the crystallite sizes decreased in thin film sample than monolith one from 44 to 31 nm by triply doped them with Er3+, Yb3+ and Sm3+ ions. It is well known that the absorption cross-section of Er3+ and Sm3+ ions is small, for that Yb3+ ions are used as a sensitizer to increase the Er3+ and Sm3+ ions PL emission and offering good spectral overlap with Er3+ and Sm3+ transition, thus allowing efficient Yb3+-Er3+-Sm3+ energy transfer with subsequent Er3+ emission.