Received 25 October 2015; accepted 28 November 2015; published 2 December 2015


1. Introduction
Herbal drugs are manufactured from fresh or dried plants having interrelated compounds that work together. In comparison pharmaceutical drugs are solitary compounds that are often synthetic in nature. Herbal drug expert treated their patient using herbs, food and lifestyles modifications to cure through addressing the root cause of illness; however modern medicine usually manages only the symptoms of diseases with strong compounds that they contain. They seldom address the actual cause of ailment. It means that herbals treatment follows relatively holistic approach for remedial purposes [1] [2] .
Ethno-pharmacological research on medicinal plants can promote the development of newer and safer compounds. These studies may help in the discovery of a new drug molecule from the herbal source and hence may endorse the use of traditional drugs in humans.
Medicinal plants have strong historical background as remedy for different ailments. Around 25% of our current prescription medicines have been originated from medicinal plants. These products have been used as medication and dietary supplements in both developing and developed countries [3] . In several countries, herbal drugs are still the most common available health source for the majority of the population and its use has been encouraged by the government authorities along with modern medicine [4] [5] .
Herbal medicines have been investigated under strict control and approved by the health authorities of developed countries worldwide. They are generally treated as dietary supplement and World Health Organization has given separate guidelines to ensure their safety and efficacy [6] [7] . However in developing countries, herbal medicines are used for the management of various disorders and their use is generally accepted in the society as alternate system of medicine [8] [9] . Herbal drugs therefore have potential for improving general health and lower health care cost [10] .
Pain can be defined as a stressful sensation in a specific part of the body. International Association for the Study of Pain defines it as “an unpleasant sensory and emotional experience related with real or probable tissue injury” [11] .
Pain encourages the person to avoid bothersome situations, to care for a wounded body part until healing and to escape related incidences in future. Most pain subsides quickly as the painful stimulus is removed and the body has repaired, but sometimes may remain regardless of elimination of the stimulus and obvious healing of the body part. Occasionally pain begins in the absence of any detectable stimulus, injury or disease.
Generally pain has been classified in three classes: nociceptive, inflammatory and pathological pain caused by damage to the nervous system or by alteration in its function e.g. fibromyalgia, irritable bowel syndrome and tension type headache. The pain pathway usually involves transduction, transmission, modulation and perception [12] .
Drugs commonly in use for pain management and are classified as opioids analgesics, non-steroidal anti-inflammatory drugs (NSAIDs) and steroidal anti-inflammatory medicines. All these medicines have adverse effects e.g. dependence, constipation and respiratory problems with opioids [13] , gastrointestinal risk and renal insufficiency with NSAIDs and immune system depression, increasing chances of infection and decreasing wound healing by steroidal treatment [14] [15] .
Medicinal plants have been extensively used for a long time to avoid these adverse effects. It is necessary to discover new plants to come up with naturally safe and effective pain killer therapy. Plants exemplify still a large available and unexplored source of new chemicals that can help in the development of novel analgesic drugs [16] [17] .
T. chebula belongs to family Combretaceae and is generally recognized as Harad. It is native to South Asia especially in India, Pakistan, Nepal, Sri Lanka, Malaysia, Vietnam and South East China. The fruits are generally smooth or frequently ridged and yellow to orange brown in color. Its principle constituents are chebulagic, chebulinic acid, anthraquinones, tannic acid, fixed oils and corlagin [18] [19] .
Traditionally T. chebula has been widely employed as carminative, expectorant, laxative and tonic agent [20] . It is also used for treating arthritic disorders, inflammation, tumor, chronic and recurrent fever. The water extract from dried T. chebula has numerous pharmacological actions including antioxidant activity [21] [22] , antibacterial [23] - [25] , antifungal [26] , antiviral [27] and anti-mutagenic activities [21] . Moreover, the oral administration of the T. chebula did not produce any toxicity in rats [28] .
Hence the goal of the present study was to assess analgesic response of methanol extract of T. chebula using tail flick test and acetic acid induced writhing test in albino mice. Standard drug aspirin was used as positive control along with negative control (saline) to evaluate and quantify the observed results.
2. Materials and Methods
2.1. Preparation of Plant Extract
T. chebula dried fruit was procured from local herbal store and identified by Prof. Dr. Shahida Siddiqui, Department of Pharmacognosy, University of Karachi and specimen voucher number issued. One kg dried fruits were crushed and soaked in 2500 ml absolute methanol for six weeks. Then soaked material was filtered through Whatman filter paper #1 and subjected to rotary evaporator under reduced pressure for removal of solvent. Leftover syrupy residue was dissolved in small quantity of water and subjected to freeze drying. Freeze dried material was stored in small glass bottles and kept in freezer at −4˚C for evaluation.
2.2. Selection of Animals
Board of Studies, Department of Pharmacology, University of Karachi permitted the study after reviewing ethical issues. Seventy five healthy albino mice of either sex weighing from 25 to 30 grams were used for study. Animals were housed in individual cages, with 12 h of light/dark cycle under controlled condition of temperature (23˚C ± 2˚C) and humidity (50% - 60%). The animals were fasted overnight but allowed fresh water ad labitum.
2.3. Experimental Design
Animals of either sex were randomly arranged into seven groups for each experiment. Three served as treated, one control and three standard groups. Each group comprise of 5 animals. Lyophilized plant material and Aspirin in doses of 300, 500 and 1000 mg/kg were homogenized in 1.5% aqueous solution of gum tragacanth separately and administered orally to mice. Control group was only administered with 1.5% aqueous gum tragacanth solution by weight (10 ml/kg). Before dose administration, general health of all animals was monitored during the acclimatization period under the laboratory environment for 7 days specially noticing diarrhea, edema, ulceration and lack of activity.
2.3.1. Tail Flick Test
In current study analgesia was assessed by tail flick latency difference (TFLD) i.e. latency of mice to remove its tail clearly out of water at 51˚C. Analgesia is loss of sensitivity to harmful stimuli with consciousness or the loss of ability to respond the painful stimulus. Different kinds of harmful stimuli can be used to induce pain such as thermal chemical and mechanical. Analgesia was actually assessed in the study according to the method of Luiz [29] . Mice were kept in a specific restrainer with only tail extending out. Then one third of the tail was submerged in thermostatically controlled water bath maintained at 51˚C. The time in sec taken to withdraw the tail clearly out of the water was noted as the reaction time.
Methanol Plant extract and Aspirin in doses of 300, 500 and 1000 mg/kg suspended in 1.5% gum tragacanth solution were given by oral route using oral intubation tube for exact dosing. Control group only received the vehicle orally. The first reading was taken immediately before administration of the test drugs and 60, 90, 120, 150, 180 and 210 minutes after the administration. The total three readings were recorded each time for each animal. Data obtained was then analyzed using mean values of different groups and calculated the mean increases in latency following drug administration.
2.3.2. Acetic Acid Induced Writhing Test
Acetic acid induced writhing test was performed to assess systemic analgesic activity as described by Koster [30] . Mice were divided into seven groups. One group served as control and given gum tragacanth as vehicle 10 ml/kg, while other six groups received plant extract and aspirin suspended in vehicle through oral route in the doses of 300, 500 and 1000 mg/kg; after 30 min 0.9% acetic acid in the dose of 10 ml/kg was administered through intra peritoneal route. Animals were then immediately placed in a transparent plastic box (23 cm Length × 12 cm Width × 13 cm Height) and number of writhing movements consisting of contraction of the abdominal muscles, drawing up of hind limbs toward the abdominal walls, stretching of hind limbs and periodic arching of the body were counted for twenty minutes, finally percent inhibition of writhes were calculated.
2.4. Handling of Data
Inhibition of writhes caused by different doses of T. chebula and Aspirin following acetic acid administration were analyzed using the following formula.
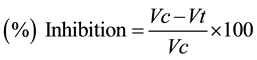
where
Vc = mean value (number of writhes for control animals);
Vt = mean value (number of writhes for treated animals.
2.5. Statistical Analysis
All the data obtained was shown as the mean and standard error to the mean (S.E.M.) and analyzed using Student t test for unpaired data and p values were observed. Results were considered significant if p values were less than 0.05 and highly significant if p value were less than 0.005 [31] .
3. Results
3.1. Tail Flick Test
The results of the analgesic activity of plant extract by tail flick test are depicted in Table 1. The results are indicated as Analgesia TFLD or Mean increase in latency after drug administration ± S.E.M in the term of sec. Results show that the T. chebula possessed varying degree of analgesic activity as compared to standard drug aspirin.
Table 1 summarizes the results of T. chebula extract at 300, 500 and 1000 mg/kg against similar doses of Aspirin and control groups. It was revealed that at 300 mg/kg the analgesic effects of extract were significant at 30 to 150 minutes and highly significant at 180 and 210 minutes as compared to aspirin which was significant at 150, 180 and 210 minutes.
Similarly the effects of T. chebula extract at 500 mg/kg were highly significant at 30 to 210 minutes as compared to aspirin which was significant at 30 minutes and highly significant at 60 to 210 minutes.
Whereas the effects of T. chebula extract at 1000 mg/kg were highly significant at 30 to 210 minutes which were similar to the effects of aspirin at 30 to 210 minutes.
3.2. Acetic Acid Induced Writhing Test
The results of the analgesic activity of plant extract by acetic acid induced writhing test are depicted in Table 2. Aspirin (positive control) and test drug T. chebula methanol extract produced significant dose dependent inhibition in number of writhes following intra peritoneal (I/P) injection of 0.9% Acetic acid solution.
![]()
Table 1. Analgesic effect of T. chebula and Aspirin in tail flick test.
n = 5. Average value ± S.E.M. *p ≤ 0.05 significant. **p ≤ 0.005 highly significant.
![]()
Table 2. Analgesic effect of T. chebula and aspirin in acetic acid induced writhing test.
n = 5. Average value ± S.E.M. *p ≤ 0.05 significant. **p ≤ 0.005 highly significant.
Aspirin at the dose of 300, 500 and 1000 mg/kg reduce the mean number of writhes to 9.1, 7.5 and 5.8 respectively (representing 35.4%, 46.8% & 58.8% inhibition) as compared to 14.1 writhes of control animal.
T. chebula extract produced significant dose related inhibition in mean number of writhes in all treated groups as compared to the positive and negative controls. Maximum effects of the T. chebula extract was observed at 1000 mg/kg in which number of writhes reduced from 14.1 to 5.2 indicating about 63.1% decrease.
4. Discussion
Herbs have been used since ages as remedies for the management of wide variety of diseases. They have a key role in world health care. Instead of the abundant achievements being made in modern medicine recently, herbal drugs still make an important involvement in health sector. W.H.O confirmed that around 80% of the world’s population especially living in developing countries depends on herbal medicines to some extent for primary health [8] [10] .
It is also projected that about one quarter of all modern medicines are directly or indirectly derived from plants origin [32] , and hence herbal drugs still possess strong potential as sources for new lead structures and for the development of standardized phytotherapeutic agents with proven efficacy and safety [33] .
The drug under investigation, T. chebula has been stated for carminative, expectorant, laxative and tonic effects [21] [22] . T. chebula has documented history for its use in the management of arthritic disorders, inflammation, tumor, pain, chronic and recurrent fever in ayurvedic and naturopathy. Moreover, its use as herbal drug is also associated with positive effects on longevity, immunity and increasing body resistance against diseases [24] [25] .
The analgesic effects of T. chebula methanol extract were analyzed using two animal models against aspirin since it is a well-known and remarkable analgesic agent [34] producing its effect through the inhibition of cyclooxygenase enzyme (COX) thus decreasing prostaglandin synthesis. Reduction of prostaglandin reduces inflammation, pain and fever. The action continues until the enzyme cyclooxygenase is regenerated in the platelets [34] .
In tail flick latency difference T. chebula extract showed significant analgesic activity at all three doses; however, the most profound effects were produced at 1000 mg/kg. The peek effect has been observed at +150 minutes after which the effects start decreasing.
Acetic acid induced writhing is a well-known technique employed of visceral pain model in rodents [35] . It depends on the administration of intense chemical stimulus that provokes nociceptive response of small duration. Intra-peritoneal injection of acetic acid causes the release of mediators of inflammation (histamine and bradykinin) that stimulate nociceptive nerve fibers. These fibers transmit information to the spinal cord and the higher centers of brain which integrate and modulate pain [36] .
In acetic acid induced writhing test, T. chebula extract demonstrated significant reduction in an average number of writhes at all three dose levels against negative control group; however maximum activity of the T. chebula extract was also observed at 1000 mg/kg in which the number of writhing movement reduced to 5.2 indicating about 63.1% decline.
Peripheral analgesics prevent the activation expressed by sensitized nociceptors [34] . Non-steroidal anti-in- flammatory drugs like aspirin inhibit synthesis of prostaglandin resulting in the decrease sensitivity of the nociceptors to pain producing agents such as bradykinin [36] . Results demonstrate that T. chebula induced a profound analgesic effect since inhibited writhing response in mice; hence it may be suggested that this may be due to inhibition of prostaglandin synthesis.
T. chebula contains tannins, chebulic acid, anthraquinones, ellagic acid, gallic acid and resin [18] . T. chebula has been commonly used to reduce inflammation [37] . Above findings suggest that T. chebula possesses analgesic activity probably by inhibiting inflammatory mediators (histamine, serotonin, bradykinin and prostaglandin) at both central and peripheral sites. However mechanism based studies on a large number of animals are essentially required to confirm the efficacy of T. chebula.
NOTES
![]()
*Corresponding author.