The Diversity of Chrysophycean Algae in an Arctic Zone of River and Sea Water Mixing, Russia ()
1. Introduction
Chrysophyte algae (Chrysophyta) are an important component of phytoplankton that is highly responsive to changes in the aquatic environment [1] . They mainly inhabit cold, fresh waters [2] - [4] , but there is evidence that many species are tolerant of high salinity levels [1] [5] [6] . In Arctic regions, species are known that occur both in fresh and salt waters, e.g. Paraphysomonas imperforata and Mallomonas tonsurata; brackish waters of Greenland are inhabited by Paraphysomonas vestita and Synura petersenii [7] .
In recent years, a number of studies have been performed on water bodies of the Arctic zone, where the climate is severe with long, cold winters and a short open-water period. In the majority of lakes studies, this period is only 2 - 3 months, and the lakes are oligotrophic or even ultraoligotrophic. Chrysophytes are proved to be a dominant group of phytoplankton in many of these lakes [7] - [18] , accounting for up to 60% - 80% of its total abundance and 50% - 70% of total biomass [18] . The highest diversity of chrysophytes is observed in the region of Bol’shezemelskaya tundra: 95 species and infraspecific taxa, including 46 species of the genus Mallomonas and 16 species of the genus Dinobryon. The authors attribute this to favorable conditions for the development of chrysophytes in lakes of the above region: water temperature 12˚С - 19˚С, pH 5.5 - 7.5, low specific conductance (19 - 59 μS∙cm−1), and the absence of competition with cyanobacteria [11] [13] [19] . Analyzing the taxonomic diversity of phytoplankton in oligotrophic lakes of the Polar Urals, Voloshko [12] [14] [20] [21] recorde 47 chrysophyte species, with representatives of the genus Mallomonas (20 species) also holding a leading position. The pH of the water during the study period is almost neutral (pH 6.7 - 7.3). In Swedish Lapland, the chrysophyte flora of lakes and pools is found to consist of 28 species from 5 genera, with the prevalence of Mallomonas (16 species) and Synura (5 species) [17] . A total of 23 chrysophyte species are recorded in fresh water bodies of the Taimyr Peninsula located between the Yenisei Gulf (the Kara Sea) and Khatanga Gulf (the Laptev Sea), which are close to the region of our study [8] . The list of chrysophytes previously found in the Khantay Reservoir on the Yenisei River, which flows into the Kara Sea, comprises 67 species, including 18 species of the genus Dinobryon Ehrenb [22] .
In this study, electron microscopic methods are used to evaluate the diversity of chrysophytes depending on hydrochemical parameters in the zone of mixing of Yenisei River and Kara Sea waters.
2. Material and Methods
During an expedition carried out in September 1-24, 2009, samples of phytoplankton and water were taken at 33 stations located in the lower Yenisei (st. 24-34), Yenisei Gulf (st. 1-4, 10, 22, 23), Gydan Bay (st. 11-19), and the Kara Sea shelf (st. 5-9, 20, 21) (Figure 1, Table 1) within the RAS Presidium Program “Integrated Research of Arctic shelf”, project 20.7.
Water samples for chemical analysis were cleared of suspended matter by filtering through membranes with a pore size of 0.45 µm (VladiSart, Russia). Nutrient elements were determined by colorimetric methods [23] ; cations, by atomic absorption and flame emission spectroscopy [24] ; anions, by HPLC [25] . Biogenic elements were determined by colorimetric methods [23] [24] . Nitrate nitrogen was determined by HPLC, nitrites―with Griss reagent; ammonium nitrogen―with indophenol method; total phosphorus―with potassium persulphate (burning at 150˚С); the determination, like with mineral phosphorus, was finished by Denigès-Atkins method. Dissolved silicic acid was determined using a yellow silica-molybdenum unit [24] .
Qualitative samples of phytoplankton were collected with a Juday plankton net (nylon fabric mesh No. 70) and fixed with 70% ethyl alcohol. Scaled chrysophytes were identified and analyzed using scanning and transmission electron microscopy (SEM and TEM). Samples for SEM were concentrated on membrane filters with a pore diameter of 1.2 µm (Millipore, United States), sputter-coated with gold, and analyzed under a Philips 525 M scanning electron microscope. In case of TEM, a concentrated sample was placed onto a Formvar-coated grid and examined under a LEO 906E transmission electron microscope. A total of 66 samples were analyzed. Sørensen’s coefficient of similarity was calculated as described [26] in order to determine the degree of difference with water bodies of Bol’shezemel’skaya Tundra, Taymyr Peninsula and Swedish Lapland.
Sørensen’s coefficient of similarity was determined by formula:
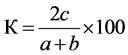 ,
,
where с is the number of common species; а is the number of species in the water bodies of ecosystems studied;
![]()
Figure 1. Schematic map of the study region and locations of sampling stations.
b is the number of species in the water bodies at the compared territories.
Floral link coefficient is calculated by the formula:
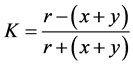 ,
,
where x is the number of specific species in the water bodies of studied ecosystems; y is the number of specific species in the water bodies at the compared territories; r is the number of common species [26] .
3. Results
3.1. Characteristics of the Study Region
Conditions of formation, hydrophysical parameters, and chemical composition of water masses in the study region are diverse (Table 1). Water depths are relatively shallow (at most 29 m), especially in Gydan Bay (2 - 11
![]()
![]()
Table 1. Coordinates of sampling stations and some physicochemical parameters of water in the study region (2009).
m). The salinity of surface waters in the study period varied from 0.03 ‰ in the southern part of Gydan Bay to 5.7‰ in its northern part and in the Kara Se shelf. Water temperature in the lower Yenisei varied between 9.2˚C and 10.8˚С, gradually decreasing to 8.2˚C - 8.6˚С in the Yenisei Gulf and Kara Sea shelf. Water temperature in Gydan Bay was almost uniform throughout its area (9.6˚С - 10.6˚С). Dissolved oxygen reached a peak of 11.7 mg∙l−1 in the lower Yenisei, varied between 10.3 and 10.9 in Gydan Bay, and decreased to 8.7 mg∙l−1 in the coastal sea waters. Water pH values were fairly high, from 7.55 in the lower Yenisei and Gydan Bay to 8.2 in the Yenisei Gulf. The concentration of dissolved silicon was the lowest in Gydan Bay (0.10 - 0.15 mg∙l−1), slightly varied along the river segment (from 2.0 to 2.2 mg∙l−1), and increased to 2.7 - 3.3 mg∙l−1 in the Yenisei Gulf. Minerl phosphorus concentration gradually increased from 2 to 15 µg∙l−1 along the river segment and varied between 5 and 13 µg∙l−1 in water areas of Yenisei Gulf and Gydan Bay.
3.2. Species Diversity
As noted previously [26] , the composition of phytoplankton in the lower Yenisei River, Yenisei Gulf, Gydan Bay, and Kara Sea shelf was dominated by diatoms, which accounted for 40% to 90% of the total species number. The diversity of planktonic chrysophytes was significantly lower, and their contribution to the species list was no more than 10% - 25% [26] . In this study, we recorded 43 chrysophyte species and infraspecific taxa. These were mainly widespread and cosmopolitan representatives of the genera Mallomonas (13 species), Spiniferomonas (6), Dinobryon (5), Synura (4), Kephyrion (5), Paraphysomonas (3), Stenokalyx (1), Chrysosphaerella (3), and Pseudokephyrion (1) (Table 1).
3.3. Fresh Waters
In the lower Yenisei (st. 24-34), chrysophytes were represented by 27 species. The group of often found species included Spiniferomonas takahashii, S. trioralis, Paraphysomonas gladiata, P. vestita, Synura petersenii, Mallomonas acaroides and M. crassisquama were also often found at st. 1 and 25 (Table 2, Figure 2, Figure 3). Dinobryon sociale and species of the genera Pseudokephyrion and Kephyrion were common components of plankton samples, as was previously described for other Arctic water bodies [18] [21] .
The chrysophyte community in the freshwater part of Gydan Bay (st. 11-19) consisted of 25 species, mainly cosmopolitan and widespread. The most frequent species were Spiniferomonas serrata, Mallomonas crassisquama, Synura petersenii, and Chrysosphaerella brevispina. The genus Spiniferomonas was better represented
![]()
![]()
Table 2. Species composition of chrysophytes in the lower Yenisei River, Yenisei Gulf, Gydan Bay, and coastal waters of the Kara Sea.
in the bay (seven species) than in the lower Yenisei, Yenisei Gulf, and Kara Sea coastal waters; species of the genera Kephyrion and Pseudokephyrion were also regularly found in plankton samples. Species such as M. heterospina, M. akrokomos and M. elongata were recorded at station 18, where the species diversity of chrysophytes was the highest (Table 2, Figure 2, Figure 3).
3.4. Mixed Waters
The diversity of chrysophytes in the Yenisei Gulf (st. 1-4, 10, 22, 23) was slightly lower (24 species). As in the lower Yenisei, the group of most frequent species included Spiniferomonas takahashii, Synura petersenii f. petersenii, S. petersenii f. kufferathii, and, to a lesser extent, Mallomonas acaroides, M. alpina, M. crassisquama and Paraphysomonas gladiata. Some chrysophytes recorded in the gulf―Synura echinulata, S. splendida (st. 10), and Chrysosphaerella coronacircumspina (st. 23, 10)―did not occur at other stations, including those in the river segment. Only a few samples contained species such as P. vestita (st. 2), M. akrokomos (st. 1), M. heterospina (st. 3), S. bourrellyi (st. 4). Chrysophytes of the genus Dinobryon occurred mainly in fresh waters of the lower Yenisei and Gydan Bay, with only single D. cylindricum cells occurring in samples of mixed waters from the Yenisei Gulf (Table 2, Figure 2, Figure 3).
![]()
Figure 2. Scales of chrysophytes of the genus Mallomonas (SEM): (a) M. alpina, scale; (b) M. crassisquama cell and (c) scale; (d) M. acaroids, scale; (e) M. caudate, scale; (f) M. costata, scale; (g) M. striata var. Striata, scale; (h) M. tonsurata, scale; (i) M. punctifera, scale; and (j) cell armour; (k) M. heterospina, scale; (l) M. multiunca var. Pocosinensis, scale.
Samples from stations in the Kara Sea shelf (st. 5-9, 20, 21) contained a total of 19 chrysophyte species, most of them widespread and cosmopolitan (Synura petersenii f. petersenii, S. petersenii f. kufferathii, Mallomonas crassisquama, etc.). In addition, a rare form such as M. multiunca var. pocosiensis was recorded there (Table 2, Figure 2, Figure 3).
3.5. Influence of Physicochemical Factors
As follows in Table 2, the species diversity of chrysophytes in the study region was dependent mostly on water salinity: it reached a maximum of 19 species at st. 18 in Gydan Bay, where salinity was the lowest (0.03‰), and remained fairly high (12 species) at st. 4 in the Yenisei Gulf (3.8‰ salinity), but markedly decreased in the Kara Sea shelf (st. 5-8, 9), along with increase in salinity and decrease in dissolved oxygen level. Our previous data
![]()
Figure 3. (a) Spiniferomonas trioralis, plate scale; (b) S. takahashii, cell armour and spine scales; (c) Paraphysomonas vestita, plate scale; (d) P. vestita cell with plate and spine scales; (e) P. gladiata, plate and spine scales; (f) Chrysosphaerella coronacircumspina, a plate scale and the base of a spine scale; (g, h) scales of Synura petersenii f. petersenii; (i) S. petersenii cell armour; (j) S. petersenii f. kufferathii scale; (k) S. spinosa, a fragment of lorica; (l) Kephyrion spirale lorica. (a, c, g) ТЕМ; (b, d-f, h-l) SEM.
[27] show that facultative marine diatoms of the genus Thalassiosira begin to prevail in the phytoplankton of this region. Samples from st. 22 with water salinity was the highest (8‰), proved to contain chrysophytes such as Mallomonas crassisquama, Synura pertersenii, Paraphysomonas gladiata, and Spiniferomonas takahashii. Several chrysophyte species (Paraphysomonas imperforata, Mallomonas tonsurata, Paraphysomonas vestita and Synura petersenii) were previously found in brackish-water lakes of Greenland [7] .
![]()
![]()
Table 3. Characteristics of geographic distribution (G) of chrysophytes (wc, widespread and cosmopolitan species; lr, species with limited distribution and rare species; a-a, arctalpine species) and their ranges of water physicochemical parameters in the zone of mixing of Yenisei River and Kara Sea.
Most chrysophytes concentrated in water layers rich in oxygen. Their diversity was the highest at st. 18, where the dissolved oxygen level reached 11.1 mg∙l−1, but sharply decreased at st. 7, where this level was low. It is noteworthy that samples from the latter station contained species such as Mallomonas akrokomos, M. striata, Synura petersenii and Spiniferomonas takahashii.
Water pH is an important factor governing the distribution of chrysophytes, and its role has been well studied. As shown previously, the optimum for the development of these algae in water bodies of northern Russia is at pH 5.5 - 7.5 [14] , while the number of chrysophyte species in the tributaries of Lake Ladoga in the spring― summer season reaches a peak at pH 7.5 - 7.92 [26] . We found that the diversity of chrysophytes was maximum at st. 18 in Gydan Bay, where water pH was 7.57, and decreased in waters with higher pH values. The literature provides detailed data on the response to pH of species from the genera Mallomonas and Synura [1] [28] - [30] . In particular, M. akrokomos and М. caudata are tolerant of wide pH range (4.0 - 9.0 and 4.5 - 9.0, respectively), and the same is true of Synura petersenii (pH 3.0 - 9.0). In the region of our study, the development of these species reached a peak at pH 7.57 - 8.09 and 7.95 - 8.09, respectively. It is considered that Mallomonas acaroides in nature prefers alkaline waters with pH > 8.0 [28] [29] . Our study confirmed the occurrence of this species in samples from waters with pH 8.2, which also contained Chrysosphaerella brevispina, Mallomonas alpina, Paraphysomonas gladiata, P. vestita and Spiniferomonas trioralis. At the lowest pH (7.55), species such as M. striata was recorded (Table 3).
It is known that even slight water turbidity leads to reduction of the species composition of planktopnic algae [31] . According to our data, however, this factor had no significant effect on the diversity of chrysophytes, except for the situation at st. 19 in Gydan Bay: water turbidity at this station was increased, and only eight chrysophyte species were recorded there, compared to a maximum of 19 species at the neighboring st. 18 (Table 1, Table 2).
An increase in the concentration of mineral phosphorus and consequent eutrophication of water bodies lead to reduction of chrysophyte species diversity. Species prevailing in waters with high phosphorus concentrations included Chrysosphaerella coronacircumspina, Kephyrion spirale, Dinobryon cylindricum, Synura petersenii, Paraphysomonas gladiata and Spiniferomonas takahashii (Table 3). Fluctuations of water temperature were minor and had no significant effect on chrysophyte species diversity in the study region.
Synura petersenii and Spiniferomonas takahashii, the most abundant and frequent species in the lower Yenisei and Yenisei Gulf, proved to successfully develop in a wide range of ecological (environmental) factors (Ta- ble 2).
Appropriate habitat conditions were also determined for rare species such as M. elongata (st. 18), M. multiunca var. pocosiensis (st. 9, 33), S. petersenii f. kufferathii (st. 2-4, 8, 9, 24, 25, 30), and P. gladiata (st. 1, 3, 4, 5, 18, 19, 22-34) (Table 3). Mallomonas elongata occurred at st. 18, in waters with the lowest salinity and pH; M. multiunca var. pocosiensis, at two stations in the lower Yenisei (st. 33) and Kara Sea shelf (st. 9) where water pH was the highest; and S. petersenii f. kufferathii, at 18 stations with increased water pH and salinity. According to Voloshko et al. [26] , S. petersenii f. kufferathii in Lake Ladoga successfully developed at water pH of 6.75 to 7.31 and mineral phosphorus content of 15 - 85 µg∙l−1, whereas the upper limit of pH range for this species in the study region extended to pH 8.11, mineral phosphorus content of 6 - 13 µg∙l−1. Paraphysomonas gladiata in our study was recorded at 17 stations, in a wide range of salinity and pH, and showed preference for waters rich in dissolved oxygen. Its development in Lake Ladoga was observed at pH 7.18 - 7.92 and 0 - 19 µg∙l−1 of mineral phosphorus [26] , but our data show that this species occurs in waters with higher values of these parameters (Table 3).
3.6. Comparison of Species Composition
Comparisons of chrysophyte species composition between sampling stations showed that representatives of the genus Mallomonas were the most diverse group of these algae (Figure 4), as was also observed in other Arctic regions [4] [7] [11] [12] [17] . This genus comprises boreal and cosmopolitan species, most of them being oligosaprobiontic and indifferent to water salinity. Species of the genera Dinobryon and Kephyrion prevailed in fresh waters, at the mouths of tributaries flowing into the lower Yenisei and at coastal stations in Gydan Bay (Figure 4).
An analysis of chrysophyte species composition between the study region and some other regions with similar climatic conditions revealed 81% similarity (Sørensen’s coefficient) of chrysophyte flora in the lower Yenisei to that in some water bodies of the Taimyr Peninsula [8] .
4. Conclusions
Analysis of phytoplankton samples from the study region by electron microscopic methods (SEM and TEM) allowed us to identify scaled chrysophytes from nine genera―Mallomonas, Spiniferomonas, Chrysosphaerella, Paraphysomonas, Synura, Dinobryon, Pseudokephyrion, Kephyrion, and Chrysococcus―and thereby expanded the species list of these algae in the Arctic zone. Some rare representatives of Chrysophyta were discovered in the samples, such as Synura petersenii f. kufferathii and Paraphysomonas gladiata previously found in lakes of
![]()
Figure 4. Changes in chrysophyte species composition depending on water salinity in the study region.
Bol’shezemel’skaya tundra [11] and Taimyr Peninula [8] and Mallomonas multiunca var. pocosiensis described from Northern California [32] .
The taxonomic composition of Chrysophyta in the study region was similar to that in some water bodies of the Taimyr Peninsula. Their species diversity depends on water salinity, being higher in fresh or freshened waters. A major proportion of chrysophytes in the region (more than 10 species) inhabit water with the following parameters: salinity 0.03‰ - 3.8‰, pH 7.57 - 8.06, turbidity 5.85 - 71.54 mg∙l−1, mineral phosphorus 0 - 0.013 mg∙l−1, dissolved oxygen 10.4 - 11.7 mg∙l−1. Synura petersenii and Spiniferomonas takahashii were proved to have the widest ecological range.
Acknowledgements
We are grateful to G. I. Popovskaya, N. A. Bondarenko, and L. N. Voloshko for their valuable comments on the manuscript.
This study was performed using the facilities of the Instrumentation Center for Electron Microscopy at the Integrated Center of Ultramicroanalysis (Limnological Institute, Siberian Branch, Russian Academy of Sciences). Expedition research and hydrochemical analysis were supported by “Complex Researches of the Arctic Shelf” the Presidium of the Russian Academy of Sciences (project No. 23.6). Analysis of chrysophytes was performed according to the budged-funded program of the Limnological Institute (project No. VI. 50.1.3).
NOTES
*Corresponding author.