1. Introduction
Cu(In,Ga)Se2 is one of the most promising semiconductors for the absorber-layer of thin-film solar cells [1] . The conversion efficiency of such cells on glass substrates is approaching 20% [2] . Some studies have shown that higher efficiency is reached in samples where a Cu(In1−xGax)3Se5 phase is formed at the surface of the CuIn1−xGaxSe2 layer [3] [4] . Therefore, CuIn3Se5 has been of interest to many groups [4] - [6] . In this work crystals of CuIn3Se5 (when x = 0) were grown by the horizontal Bridgman methods using a direct combination of high purity 5N for Cu, 6N for Se and Ga. The elements were placed in a quartz tube sealed under a vacuum of 5 × 10−6 Torr. This tube was placed on a horizontal furnace, for 48 hours, at a temperature higher than the melting temperature of the compound and then left to slowly cool down. Energy Dispersive Spectrometry (EDS) and X-Ray Diffraction (XRD) were used to calculate the compositions of the ingots considered as very important parameters. The hot point probe method is used in order to determine the conduction types of these ingots. Photoluminescence allowed us to check their optical properties. The type of transition was determined by varying the gap energy as a function of the temperature and as a function of the excitation power.
2. Experiments
Crystals with different compositions were synthesized by direct combination of high purity 5N for Cu and In, 6N for Ga and Se in the desired proportions. The elements were placed in a quartz tube sealed under a vacuum of 5 × 10−6 Torr. This tube was placed ina horizontal heater that reached a temperature exceeding the melting point of the compound. It was left in the heater for 72 hours at which point it was allowed to slowly cool down [7] .
To characterize the crystals, X-Ray Diffraction was carried out using a Seifert MZIV powder diffractometer (q, 2q geometry) with Cu (Ka) radiation (l = 1.5406 Ǻ). The chemical composition of the obtained samples were given by
EDS
(Link type AN 1000 55/S) coupled to a scanning microscope (Cambridge type S360). The Photoluminescence (PL) measurements were performed at different temperatures (from 4.2 K to 85 K) by directly immersing the samples into liquid helium. Excitation was provided by a 632.8 nm He-He laser (20 mW). The illumination of the samples was realized using fiber optic light guides (UV-visible). A3 mm spot was focused on the sample with a power of 2 mW/cm2. The emission spectrum, collected through another fiber (visible-IR), was analyzed using a grating monochromator (30 cm focal length, 600 lines/mm, blazed at 760 nm).
3. Results and Discussion
3.1. Characterization by EDS
In Table 1 the characterization results of CuIn3Se5 materials by EDS are presented. The samples show a nearly perfect stœchiometry since the magnitude of deviation from stœchiometry, 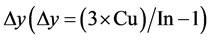 , is very small [8] . The results of the chemical compositions of a CuIn3Se5 sample have a large value of Dy (0.3). A detailed analysis is done in order to study the secondary phases that appear in this sample which will be discussed later on. The CuIn3Se5 samples present n-type conductivity.
, is very small [8] . The results of the chemical compositions of a CuIn3Se5 sample have a large value of Dy (0.3). A detailed analysis is done in order to study the secondary phases that appear in this sample which will be discussed later on. The CuIn3Se5 samples present n-type conductivity.
3.2. Characterization by X-Ray Diffraction
The spectra of different CuIn3Se5 samples obtained by X-Ray Diffraction are shown in Figure 1. These samples are well-crystallized and all existent peaks are similar to those found in previous publications [9] - [11] . Thus, our XR spectra show the presence of several preferential orientations according to planes (112), (220) and (312) for all samples. Since the characteristic peaks concerning the chalcopyrite structure were not observed, the conclusion was made that unlike the CuInSe2 compounds, the CuIn3Se5 compounds do not have a chalcopyrite structure [4] [12] . They either have a Stanite structure [13] , an Ordered Defect Chalcopyrite structure (ODC), oran Ordered Vacancy Chalcopyrite structure (OVC).
Sample (E) was studied using X-Ray Diffraction (Table 1) and was found to have bad stœchiometry as the magnitude of deviation from stœchiometry was high (Δy = 0.3). The spectrum presented in Figure 2 shows the characteristic peaks concerning CuIn3Se5 an dadditional peaks associated with other secondary phases [14] :
![]()
Table 1. Chemical compositions of CuIn3Se5 bulk samples obtained by EDS.
![]()
Figure 1. Spectrum of CuIn3Se5 obtained by X-Ray Diffraction for one sample.
![]()
Figure 2. Spectrum of CuIn3Se5 obtained by X-Ray Diffraction for sample (E). The symbol (*) indicates the InSe phase and the symbol (?) indicates the CuIn2Se3.5 phase.
Peaks located at 2θ = 11.4˚, 21.21˚, 13.04˚ and 30.96˚ indicated by the symbol (*), represent the InSe phase.
Peaks located at 2θ = 15.28˚ and 26˚ indicated by the symbol (?) can be associated with a CuIn2Se3.5 phase [15] .
The presence of these phases in the case of CuIn3Se5 is sometimes possible when CuIn3Se5 is equivalent to the CuInSe2 compound with indium excess. The a and c lattice parameters of CuIn3Se5 presented in Table 2 have been calculated from the spectra where a = 5.76, c = 11.52. These values of a and c are in agreement with those reported in Marin et al. and Negami et al. [16] [17] .
3.3. Photoluminescence
Figure 3 presents the photoluminescence spectrum of CuIn3Se5 obtained at 4.2 K. The spectrum shows the exciton position which is required to determine the gap value of 1.23 eV [14] [18] . The activation energy level implicated in the optical transition is found by subtracting the emission peak located at 1.09 eV from the gap value. In other words the activation energy is equal to 1.23 − 1.09 = 0.140 eV (140 meV). The same value has been
![]()
Table 2. Values of a, c and c/a lattice parameters of the different CuIn3Se5 samples.
![]()
Figure 3. Photoluminescence spectrum of CuIn3Se5 at 4.2 K.
found by varying the temperature of the CuIn3Se5 samples. In the case of CuIn3Se5, the fiber optic presents absorption at 1 eV, because of this the corrected photoluminescence spectrum of the fiber absorption shows two peaks.
3.3.1. Influence of Temperature
Figure 4 presents the different photoluminescence spectra of CuIn3Se5, as a function of the temperature at constant excitation intensity (114 mW/cm2). By increasing the temperature, the intensity of the emission peak decreases and a deviation toward low energies is observed.
Figure 5 shows the intensity curve of the photoluminescence signal as a function of 103/T, thus the slope of the line tangent to the curve gives the activation energy in the order of 145 meV.
The full width at half maximum (FWHM) of the peak is found to be in the order of 105 meV. This value shows that the transition is a D− A type. Since CuIn3Se5 is equivalent to CuInSe2 with an indium excess, the defects that appear are probably InCu, VCu and InSe. Defect InCu is more probable than VCu and InSe given that it has a low formation energy ε [19] - [21] 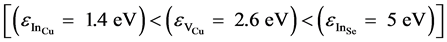 .
.
Figure 6 presents the variation of the gap energy as a function of the temperature with the condition that the activation energy remains constant. Between 4.2 K and 77 K, a decrease of the gap energy is approximately 60 meV. This variation can be expressed as the following linear equation:
Eg = 1.23 − 0.0008 T
3.3.2. Influence of the Excitation Power
In order to distinguish a D-A transition from other types of transitions, a method of increasing the excitation power and observing a shift of the peak toward high energies was used (Figure 7). When this occurs the pairs number becomes increasingly important in this transition and the remote pairs will also begin to take place in the
![]()
Figure 4. Variation of CuIn3Se5 photoluminescence spectra with tem- perature at a constant excitation power (114 mW/cm2).
![]()
Figure 5. Photoluminescence signal intensity of CuIn3Se5 as a function of 103/T.
![]()
Figure 6. Variation of the peak position (Δ) and the value of the gap (□) along with the temperature of the CuIn3Se5 sample.
transition. In a D-A type transition, it is known [22] that the signal intensity, I, depends on the power, P, according to a law given by I = C.Pa, where C and α are constant. The variation of photoluminescence spectra as a function of the excitation intensity at a constant temperature of 4.2 K (Figure 7) permits the finding of the value of α.
The variation of the light intensity of CuIn3Se5 emission peak as a function of the excitation power at a constant temperature of 4.2 K is shown in Figure 8. The value of α found is in the order of 0.76, and thus I = C.Pa can be rewritten as I = 0.028P0.76.
4. Conclusion
Firstly, samples of CuIn3Se5 have been prepared by the Bridgman method. The different samples have then been characterized by several techniques (EDS, XR, hot point probe and photoluminescence). All but one sample present good stœchiometry and are well crystallized. Their lattice parameters a and c are similar to those in previous publications, specifically c/a » 2. The characterization by photoluminescence allowed the gap value of 1.23 eV to be determined for these compounds. Studying the variation of the gap as a function of the temperature showed that the variation coefficients of the gap along with the temperature of our CuIn3Se5 compounds had the same order of magnitude. These coefficients were found to be negative for our samples. The CuIn3Se5 sample differed from the rest as it was from good stœchiometry. Further analysis of this sample showed the existence of other secondary phases (InSe and CuIn2Se3.5).
![]()
Figure 7. Variation of CuIn3Se5 photoluminescence spectra with excitation power at a constant temperature of 4.2 K.
![]()
Figure 8. Variation of light intensity of the CuIn3Se5 emission peak as a function of the excitation power at a constant temperature of 4.2 K.
Acknowledgements
The authors wish to express their sincere thanks to Marcelle Farhat for her helpful collaboration.