1. Introduction
In nature, uranium has three radioactive isotopes: 238U(99.2743%), 235U(0.7200%) and 234U(0.0057%) [1] [2] . The former two isotopes decay in the forms:
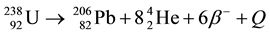
and ,
,
where Q is the heat, β denotes the beta decay and He stands for the element Helium. The decay constants λ for 238U and 235U are 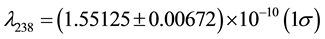 a−1 and
a−1 and  a−1, respectively [2] -[4] .
a−1, respectively [2] -[4] .
These nuclear reactions occur in host minerals, such as zircon (ZrSiO4), and are the basis of the U-Pb dating method in geology [5] -[8] . In a mineral, Pb and U isotopes obey the exponential decay law:
 (1)
(1)
and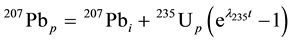 , (2)
, (2)
where the subscripts i and p represent the initial measurement time and the present, respectively, and t is the age of the mineral [1] [6] .
The coordinates n(206Pbp)/n(238Up) (n, the number of isotopes in the bracket) as the ordinate and n(207Pbp)/ n(235Up) ratios as the abscissa form the Pb/U ratio diagram (Figure 1). Samples formed t years ago plot on either the Concordia or Discordia lines [9] -[12] . For instance, the classical Discordia line was discovered by Ahrens (1955) in Zimbabwe. Equation (1) divided by 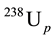 is n(206Pbp)/n(238Up):
is n(206Pbp)/n(238Up):
 . (3)
. (3)
Similarly for n(207Pbp)/n(235Up), we have
![]() , (4)
, (4)
from Equation (2).
To interpret the Discordia line, conventional theories have proposed: 1) this line was caused by Pb loss or U gain after formation of the host mineral [9] [11] -[17] , 2) the upper intersection of the Discordia and Concordia lines represents the crystallization age of the mineral [12] and 3) the lower intersection of the Discordia and Concordia lines represents the metamorphic age of the mineral [14] .
However, previous theories are not tenable when used in the following cases:
1) the lower intercept point is negative or
2) no upper intercept point exists.
For instance, in Zheng et al. (2012) (Figure 1), all zircons in YX1 from Yingxian lamproites were found to be discordant and yielded a lower intercept age of −370 ± 690 Ma. According to conventional theories, this age indicates that the samples will experience a metamorphic process in a distant age. In addition, in Zheng et al. (2012), all zircons in HBxa from Hebi basalt are also discordant, but yield no upper intercept age. According to conventional theories, these data indicate that the samples did not crystallize until the present. Apparently, the explanations do not conform to the objective facts: the samples are in front of scientists now. New studies should thus focus on resolving these discrepancies.
Herein, the slope years tslopes for the U-Pb dating method for the Concordia and Discordia lines are presented, and a method for estimating values for tslope from the experimental data is proposed. In addition, four examples are presented to illustrate the application of the proposed method.
2. Methodology
2.1. Basic Assumptions
In this study, the basic assumptions for the U-Pb dating method included the following:
a) The decay constants λ238 and λ235 are precisely determined. For instance, the decay constants in Jaffey et al. (1971) are of good quality and widely accepted. The number of citations of this paper is greater than 1200 (data from Web of Science);
b) Host minerals are not influenced by chemical reactions after formation. The minerals included apatite [18] [19] , baddeleyite [20] -[25] , monazite [26] -[33] , tantalite [34] -[38] , titanite [39] -[41] , uraninite [42] [43] and zircon [44] [45] , etc.;
![]()
Figure 1. Pb/U ratio diagram. This diagram shows the predicament for conventional theories. The Concordia (blue, colour for online version) and classical Discordia (black) for Zimbabwe samples (black diamond points) (Ahrens, 1955) are illustrated. This Discordia and Concordia intercept at A and B, for which the meanings in conventional theories are shown in the lower-right corner. Two counter-examples to traditional theories are also shown: HBxa (hexagon points and red Discordia, Zheng et al. (2012)) and YX1 (right triangle points and green Discordia, Zheng et al. (2012)). See discussions in text.
c) Present 206(7)Pbp and 235(8)Up isotope concentrations in host minerals can be precisely measured using mass spectrometry (MS). Such MS instruments include sensitive high mass-resolution ion microprobe (SHRIMP) [46] [47] , LaserProbe-inductively coupled plasma mass spectrometry (LP-ICPMS) [48] and Cameca IMS-series [44] [49] , etc.
2.2. Slope k and Slope Year Tslope
In mathematics, the variance on the ordinate is a function of the variance on the abscissa [50] . Therefore, n(206Pbp)/n(238Up) is a function of n(207Pbp)/n(235Up) in the Pb/U diagram (Figure 2):
![]() . (5)
. (5)
The theoretical expressions for this function under different conditions are given in Section 2.4.
Next, the slope k of the tangent line at point A on the general curve of Equation (5) was determined. The partial derivative of ![]() (Equation (3)) with respect to t is
(Equation (3)) with respect to t is
![]() . (6)
. (6)
Similarly, we have
![]() (7)
(7)
from Equation (4). Equation (6) divided by Equation (7) gives
![]() . (8)
. (8)
![]()
Figure 2. Pb/U ratio diagram. The general curve (in blue) for ![]() and tangent line at point A on this curve are shown. The definition of the slope at this point is also given.
and tangent line at point A on this curve are shown. The definition of the slope at this point is also given.
In this equation, the second part is the definition of the slope of the tangent line [50] :
![]() . (9)
. (9)
This equation indicates that if t is determined, the value of k is a constant (Table 1) since t ≥ 0, 0 < k ≤ 0.1575. In addition, the slope monotonically decreases with increasing time t (Figure 3).
If k is determined (see Section 2.6), the slope year is given by rewriting Equation (9):
![]() . (10)
. (10)
2.3. Initial 206(7)Pbi Concentrations in Minerals
If the values for tslope, 206(7)Pbp and 235(8)Up are known, the initial 206(7)Pbi concentrations in minerals can be determined using the following:
![]() (11)
(11)
and![]() , (12)
, (12)
which are derived from Equations (1) and (2). Clearly, the concentrations are greater than or equal to zero:![]() .
.
2.4. Mathematical Expressions for the Concordia and Discordia Lines
The initial 206(7)Pbi isotope concentrations determine the mathematical expressions for the general graph in Figure 2. This relationship can be demonstrated using assumed samples formed at the same time t with specific initial conditions. Assume there are three samples (1, 2 and 3, Figure 4(a)) with
![]() (13)
(13)
and an additional three samples (4, 5 and 6, Figure 4(b)) with
![]()
Figure 3. Plot of the slope k versus time t.
![]()
![]() (a) (b)
(a) (b)
Figure 4. Histories of Pb/U ratios (blue circle) for different samples on (a) Concordia and (b) Discordia. The red arrows indicate the direction of the evolution of each ratio.
![]()
Table 1. Values of the slope for specific years.
a, calculated from Equation (9)
![]() . (14)
. (14)
The mathematical expressions are given by solving the first-order differential Equation (9) using Equations (3) and (4):
![]() . (15).
. (15).
The solution to this equation is different for each set of samples.
a) For samples 1, 2 and 3, rewriting Equation (15) using Equation (13) gives
![]() . (16)
. (16)
The general solution of Equation (16) is
![]() . (17)
. (17)
Since the concentrations of ![]() and
and ![]() are both zero at t = 0, the result is 0 = 0 + C; thus, C = 0. Therefore,
are both zero at t = 0, the result is 0 = 0 + C; thus, C = 0. Therefore,
![]() (18)
(18)
or
![]() , (19)
, (19)
which is the expression for the Concordia line.
b) For samples 4, 5 and 6, because of the existence of the variances in ![]() and/or
and/or ![]() (Equation (14)), Equation (15) is not an elementary function and the solution to it cannot be obtained using elementary integral calculus.
(Equation (14)), Equation (15) is not an elementary function and the solution to it cannot be obtained using elementary integral calculus.
This difficulty can be overcome in the following manner. Consider a geological body (containing samples 4, 5 and 6) with continuous 206Pbi, 207Pbi, ![]() and
and ![]() distributions. Then
distributions. Then ![]() and
and ![]() in the system are continuous variables [50] . Looking back to the original differential Equation (9):
in the system are continuous variables [50] . Looking back to the original differential Equation (9):
![]() . (20)
. (20)
Since k is a constant when t is given (Table 1), the solution to this equation is
![]() , (21)
, (21)
where k and b are the slope and intercept of the line, respectively. This equation shows that the general curve in Figure 2 is a straight line, i.e. the Discordia line.
Equation (21) is consistent with the initial condition (Equation (14)). If k = 0.15751 (at t = 0) is applied:
![]() . (22)
. (22)
This equation indicates that 1) in the geological system, 206Pbi/238Ui monotonically increases with increasing 207Pbi/235Ui from samples 4 to 5 to 6 (Figure 4(b)) and 2) these two ratios for the three samples cannot simultaneously be zero.
2.5. Histories of Pb/U Ratios on the Concordia and Discordia Lines
The ![]() also determines the histories of the
also determines the histories of the ![]() data points on the Concordia and Discordia lines. In Figure 4, the histories are shown for
data points on the Concordia and Discordia lines. In Figure 4, the histories are shown for
a) samples 1, 2 and 3 (Figure 4(a)), for which when t = 0, the ![]() points plot on the origin (0, 0) where the Concordia line begins (Equation (19)). As time increases, the slope of the curve decreases from 0.15751 (0 Ma) to 0.06870 (1000 Ma) to 0.02997 (2000 Ma) and finally to 0.01307 (3000 Ma) (Table 1) and
points plot on the origin (0, 0) where the Concordia line begins (Equation (19)). As time increases, the slope of the curve decreases from 0.15751 (0 Ma) to 0.06870 (1000 Ma) to 0.02997 (2000 Ma) and finally to 0.01307 (3000 Ma) (Table 1) and
b) samples 4, 5 and 6 (Figure 4(b)), for which when t = 0 the ![]() points plot on a straight line with slope 0.15751 (Equation (22)). As time increases, the three
points plot on a straight line with slope 0.15751 (Equation (22)). As time increases, the three ![]() points plot on discordant lines with different slopes, and the slope of each line decreases from 0.15751 (0 Ma) to 0.06870 (1000 Ma) to 0.02997 (2000 Ma) and finally to 0.01307 (3000 Ma) (Table 1).
points plot on discordant lines with different slopes, and the slope of each line decreases from 0.15751 (0 Ma) to 0.06870 (1000 Ma) to 0.02997 (2000 Ma) and finally to 0.01307 (3000 Ma) (Table 1).
2.6. Methods for Determining k from Experimental Data
For n ![]()
![]() data points obtained from a mass spectrum, the k values are given as follows.
data points obtained from a mass spectrum, the k values are given as follows.
a) If the n data points plot on the Concordia line (Figure 4(a)), using Equation (19), the slope of the ith data point is
![]() , (23)
, (23)
where![]() . The mean slope for all the n points is then
. The mean slope for all the n points is then
![]() . (24)
. (24)
b) If the n data points plot on the Discordia line (Figure 4(b)), the slope can be determined using the least squares method [51] . This method gives a linear function for the points:
![]() , (25)
, (25)
where
![]() (26)
(26)
and![]() ,
, ![]() and
and![]() . See proofs for kDiscordia in Appendix A.
. See proofs for kDiscordia in Appendix A.
2.7. Error Propagation
For a function![]() , where x, y and z are independent variables, the error (1σ) is given by
, where x, y and z are independent variables, the error (1σ) is given by
![]() , (27)
, (27)
where![]() ,
, ![]() and
and ![]() are the standard errors for x, y and z, respectively [51] .
are the standard errors for x, y and z, respectively [51] .
According to Equation (27), the standard error for tslope (Equation (10)) is
![]() (28)
(28)
or
![]() , (29)
, (29)
where ![]() a−1 and
a−1 and ![]() a−1 [3] and
a−1 [3] and ![]() is the standard error of the slope. Then the values for
is the standard error of the slope. Then the values for ![]() are given as follows.
are given as follows.
a) For concordant data, the standard error of the ith slope (Equation (23)) is
![]() , (30)
, (30)
and the standard error of the mean slope (Equation (24)) is
![]() . (31)
. (31)
b) For discordant data, the standard error of k in Equation (26) is
![]() . (32)
. (32)
See proofs of this equation in Appendix A.
According to Equation (27), the standard error for 206(207)Pbi (Equations (11) and (12)) is
![]() (33)
(33)
where m and n stand for 206(7) and 235(8) respectively, ![]() and
and ![]() are taken from experimental data,
are taken from experimental data, ![]() is obtained using Equation (28) and
is obtained using Equation (28) and ![]() a−1 and
a−1 and ![]() a−1 [3] .
a−1 [3] .
3. Applications
To demonstrate the validity of our work, four examples are illustrated (Table 2 and Figure 5). Table 2 includes original Pb/U isotope ratios from the published literature along with the slope years (i.e. U-Pb ages) when the samples were formed.
The first example comes from Qinghu granite in the Nanling Range, South China [44] . The Pb/U ratios in this granite are the concordant type (Figure 5(a)) [44] . The slope and slope year were calculated using Equations (24) and (10), respectively, and found to be kConcordia = 0.13792 ± 0.00025 and tslope = 160 ± 2 Ma (Table 2), which are in good agreement with values reported by Li et al., 2009.
The k and tslope values for the three discordant examples described in the introduction were also calculated using Equations (26) and (10), respectively. For the Zimbabwe uranium deposit (Figure 5(b)), the slope was kDiscordia = 0.03950 ± 0.00178 and slope year was tslope = 1668 ± 55 Ma. For amphibolites in the Yingxian lamproite (YX1, Figure 5(c)), the slope was kDiscordia = 0.06779 ± 0.00564 and slope year was tslope = 1016 ± 100 Ma. For Hebi amphibolites (HBxa, Figure 5(d)), the slope was kDiscordia = 0.010734 ± 0.00196 and slope year was tslope = 3237 ± 220 Ma.
4. Conclusion
A method for determining the slope year for the U-Pb dating method and initial 206(7)Pb concentrations in samples was described. It was also found that if no 206(7)Pb isotopes are initially present in minerals, the Pb/U ratios plot on the Concordia line. On the other hand, if 206(7)Pb isotopes are initially present in minerals, the Pb/U ratios plot on the Discordia line. Therefore, the Discordia line is not the result of Pb loss or U gain. Furthermore, methods for determining the slope year using experimental data were also proposed and applied to data on four samples previously described in the literature. These results demonstrate that our approach is useful for geological research.
![]()
![]()
Table 2. Values for 206Pb/238U, 207Pb/235U, the slope (k) and the slope year (tslope) of zircons in different geological bodies. The Pb/U isotope ratios in the Qinghu granite (07QH-1), a Zimbabwe uranium deposit, Yingxian amphibolites (YX1) and Hebi amphibolites (HBxa) are taken from Li et al. (2009), Ahrens, (1955), Zheng et al. (2012) and Zheng et al. (2012), respectively.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 41303047, 90914010 and 41020134003).
Appendix A: Standard Error (1σ) for the Slope Using the Least Squares Method
The least squares method is described in textbooks on probability statistics [51] [52]. For a measured set of values (x1, y1,) … (xn,yn), there is a line:
![]() (A.1)
(A.1)
that best fits the data. The quality of this line is determined by
![]() . (A.2)
. (A.2)
When ![]() is at its minimum value, the estimation (Equation (A.1)) is the “best” fitting of the measured data. This approach is referred to as the method of linear-least-squares.
is at its minimum value, the estimation (Equation (A.1)) is the “best” fitting of the measured data. This approach is referred to as the method of linear-least-squares.
To find the minimum value for![]() , the following equation must be solved:
, the following equation must be solved:
![]() (A.3)
(A.3)
giving
![]() , (A.4)
, (A.4)
where ![]() and
and![]() . Then Equation (A.1) becomes
. Then Equation (A.1) becomes
![]() . (A.5)
. (A.5)
The variance of a new predicted ![]() then follows:
then follows:
![]() , (A.6)
, (A.6)
where σ is the standard error of ![]() or
or
![]() , (A.7)
, (A.7)
if n is very small. Because k follows a Gaussian distribution, its variance is
![]() (A.8)
(A.8)
The square root of this equation is the 1σ error of k.