Zinc Chloride Catalyzed Regioselective Nitration of Aromatic Hydrocarbons Using Tetrachlorosilane-Sodium Nitrate Homogeneous System ()
1. Introduction
Nitrated aryl compounds remained indispensable over the last two centuries due to their industrial and commercial applications [1] - [3] . They are used as plastics, agrochemicals, explosives, dyes, pigments and polymers [4] . These compounds can be synthesized from aryl precursors employing various nitrating reagents (e.g., nitric acid, metallic nitrates, nitronium salts and acetyl nitrates) [5] [6] . In this context, aromatic nitration in homogeneous and heterogeneous media was the subject of many outstanding reviews and monographs [5] - [10] .
In spite of the diverse investigations performed and the progression made to the nitration process, most of these strategies are facing several drawbacks. The general keys among them are the acidic corrosivity of the nitrating agents, poor regioselectivity and environmental pollution [11] - [15] . Furthermore, nitrations are usually associated with heat which in turn may cause secondary reactions or decomposition of the final product which consequently leads to low purity and yields [16] - [19] . Moreover, redox sensitive compounds and acid-hydro- lysable groups are not eligible under these conditions [20] - [24] .
Industrially speaking, nitrations under milder conditions received much attention in order to overcome obstacles associated with the hazards of the nitrating reagents. Advances in this field included palladium catalyzed nitration, the use of nitrate salts and catalyst-free nitration of aryl boronic acids [11] [20] [22] [25] - [29] . Although these methods were successful in limiting the use of corrosive and harsh reagents, these reagents were not cheap [30] - [36] . Furthermore, these agents were difficult to prepare, store and handle. Moreover, low degrees of chemoselectivity were often associated with certain aromatic systems. In this context, there are strong stimulants for the development of continuous fixed bed catalytic methods for the regioselective nitration of aromatic hydrocarbons.
2. Material and Methods
All chemical reagents for the synthesis of compounds were purchased from Sigma-Aldrich-Fluka or Merck (AMD) and used without further purification unless stated otherwise. Unless noted otherwise, silica gel 60 (Macherey-Nagel, 50 - 200 μm) was used for column chromatography. TLC plates (silica gel 60 F254, 0.20 mm) were purchased from Merck. All melting points were determined on a Gallenkamp electric melting point apparatus. IR spectra were recorded using KBr disks with a Mattson 5000 FTIR spectrometer, Faculty of Science, Cairo University. The 1H NMR data were measured in CDCl3 or DMSO-d6 on a Varian XL 200, 300 MHz instruments using TMS as an internal standard were carried out at the Micro Analytical Center of Cairo Univ., Giza, Egypt. Chemical shifts were reported in ppm (δ) downfield from that of TMS and coupling constants are expressed in hertz. Mass spectra were recorded on GC-MS QP-1000 EX. Shimadzu Instrument (Faculty of Science, Cairo University). These spectra indicated ≥99% MS-purity of the prepared compounds.
Typical experimental procedure for the nitration of phenol: to a stirred solution of the phenol (94.11 mg, 1 mmol) in dichloromethane (10 mL), NaNO3 (84.9 mg, 1 mmol) was added. TCS (229.59 µl, 2 mmol) and ZnCl2 (13.63 mg, 10% mole) were then added and the reaction mixture was stirred at room temperature in the dark for the indicated period in Table 1. After completion of the reaction (monitored by TLC), water was added and the reaction mixture was extracted with dichloromethane (25 mL × 4) and dried over Na2SO4. The solvent was distilled off and the resulting crude product can be separated either by steam distillation or by silica gel (60 - 120 mesh) using petroleum ether-ethyl acetate mixture as eluent to give a pure compound.
Nitrobenzene [37] :
Yield = 90%, yellow colored liquid, bp 210˚C. IR (liquid film): νmax = 3108, 3078, 2935, 1620, 1607, 1521, 1382, 1363, 1347, 1317, 1308 cm−1. 1H-NMR (400 MHz, CDCl3): δH = δ 8.26 - 8.17 (m, 2H), 7.42-7.35 (m, 2H), 7.47 (t, J = 7.47 Hz, 1H), 7.62 - 7.50 (m, 2H).
1-Nitronaphthalene [38] :
Yield = 90%, yellow colored solid, mp 50˚C - 52˚C. IR (KBr): νmax = 2924, 1519, 1338, 1260, 804, 763 cm−1. 1H-NMR (400 MHz, CDCl3): δH = δ 8.43 (dd, J = 0.96, 8.64 Hz, 1H), 8.10 (dd, J = 1.21, 7.62 Hz, 1H), 7.99 (d, J = 8.21 Hz, 1H), 7.83 (d, J = 8.39 Hz, 1H,), 7.55 - 7.63 (m, 1H), 7.46 - 7.54 (m, 1H), 7.41 (t, J = 7.94 Hz,1H).
4-Nitrotoluene [39] :
Yield = 65%, yellow colored solid, mp 50˚C - 52˚C. IR (KBr): νmax = 3109, 3084, 2939, 2834, 1604, 1598, 1567, 1512, 1495, 1469, 1379, 1366, 1353, 1346, 1322, 1297, 1180, 1116, 1108, 1018, 859, 839, 786, 738, 633, 619 cm−1. 1H-NMR (400 MHz, CDCl3): δH = 8.14 (d, 2H), 7.49 (d, 2 H), 2.34 (s, 3H).
4-Nitrophenol [37] :
Yield = 55%, yellow colored solid, mp 108˚C - 110˚C. IR (KBr): νmax = 3360, 3119, 3078, 2921, 1613, 1593, 1496, 1337, 1295, 1113,864 cm−1. 1H-NMR (400 MHz, CDCl3): δH = 9.83 (s, 1 H), 8.09 (d, 2H), 7.08 (d, 2 H).
2-Nitrophenol [37] :
Yield = 35%, yellow colored solid, mp 44˚C. IR (KBr): νmax = 3240, 3113, 3091, 2924, 1617, 1589, 1534,
![]()
Table 1. Effect of solvents in the nitration of phenol with NaNO3/TCS.
a. No reaction was observed in the absence of TCS; b. The reaction was exothermic, low yielding and the formation of unresolved by-products was constantly observed; c. No reaction.
1478, 1449, 1372, 1332, 1315, 1177, 1078, 1026 cm−1. 1H-NMR (400 MHz, CDCl3): δH = 10.57 (s, 1 H), 8.10 (dd, J = 8.4, 1.7 Hz, 1 H), 7.57 (dt, J = 8.5, 8.5, 1.7 Hz, 1H), 7.16 (ddd, J = 8.5, 1.5, 0.4 Hz, 1H), 6.88 (dt, J = 8.5, 8.4, 1.5 Hz, 1H).
2-Methyl-4-nitrophenol [37] :
Yield = 90%, yellow colored solid, mp 98˚C - 100˚C. IR (KBr): νmax = 3440, 3214, 3087, 2928, 1609, 1541, 1462, 1405, 1334, 1313, 1244, 1157, 679 cm−1. 1H-NMR (400 MHz, CDCl3): δH = 7.95 (dd, J = 9.1, 4.4 Hz,, 1H), 7.90 (s, 1H), 7.09 (dd, J = 9.2, 4.7 Hz, 1H), 5.50 (s, 1H), 2.15 (s, 3H).
3-Methyl-4-nitrophenol [37] :
Yield = 90%, yellow colored solid, mp 112˚C - 118˚C. IR (KBr): νmax = 3282, 3103, 3049, 2927, 1624, 1572, 1527, 1469, 1448, 1422, 1384, 1309, 1259, 1175, 1156, 1102, 851 cm−1. 1H-NMR (400 MHz, CDCl3): δH = 8.05 (m, 1 H), 6.75 (m, 2H), 5.51 (s, 1H), 2.34 (s, 3H).
4-Nitroresorcinol [40] :
Yield = 60%, yellow solid, mp 80˚C - 82˚C. IR (KBr): νmax = 3354, 1532, 1397, 1255 cm−1. 1H-NMR (400 MHz, CDCl3): δH = 10.97 (s, 2H), 7.45 (d, J = 9.2 Hz, 1H), 6.52 (d, J = 3.4 Hz, 1H), 6.47 (dd, J = 9.2, 4.7 Hz, 1H).
2,4-Dinitroanisol [40] :
Yield = 45%, yellowish powder, mp 94˚C - 95˚C. IR (KBr): νmax = 3091, 1624, 1607, 1491, 1466, 1460, 1438, 1418, 1344, 1318, 1285, 1184, 1163, 1138, 1073 cm−1. 1H-NMR (400 MHz, CDCl3): δH = 8.703 (s, 1H), 8.4 (dd, J = 8.4, 4.8 Hz,1 H), 7.25 (dd, J = 4.7, 1.5 Hz, 1H), 4.18 (s, 3H).
2,4-Dinitrophenol [41] :
Yield = 90%, yellow crystalline powder, mp 112˚C - 114˚C. IR (KBr): νmax = 3272, 3111, 1627, 1602, 1595, 1558, 1481, 1434, 1349, 1334, 1267, 1236, 1167, 1149, 1138, 1111, 1068 cm−1. 1H-NMR (400 MHz, CDCl3): δH = 11.03 (s, 1H), 9.08 (d, J = 2.8 Hz, 1H), 8.46 (dd, J = 8.5, 4.9 Hz, 1H), 7.34 (d, J = 9.2 Hz, 1H).
1-Chloro-4-nitrobenzene [42] :
Yield = 70%, Yellow crystals, mp 82˚C - 83˚C. IR (KBr): νmax = 3106, 3069, 1604, 1578, 1519, 1477, 1424, 1403, 1383, 1358, 1344, 1314, 1238, 1175, 1109, 1095, 1013 cm−1. 1H-NMR (400 MHz, CDCl3): 8.10 (d, J = 2.5 Hz, 2H), 7.52 (d, J = 2.4 Hz, 2H).
3-Nitroacetophenone [43] :
Yield = 45%, white to light beige crystalline powder, mp 72˚C - 76˚C. IR (KBr): νmax = 2955, 2923, 2852, 1693, 1633, 1462, 1378, 1279, 1209 cm−1. 1H-NMR (400 MHz, CDCl3): δH = 8.61 (s, 1H), 8.45 (dd, J = 4.8, 1.5 Hz, 1H), 8.33 (dd, J = 4.8, 1.5 Hz, 1H), 7.82 (m, 1H), 2.50 (s, 3 H).
3-Nitropyridine [44] :
Yield = 80%, white to light beige crystalline liquid, mp 41 - 42 (lit. 120; 40 Torr)˚C. IR (KBr): νmax = 3058, 2868, 1602, 1571, 1526, 1471, 1427, 1353, 1239 cm−1. 1H-NMR (400 MHz, CDCl3): δH = 7.60 (dd, J = 8.4, 4.8 Hz, 1H), 8.53 (ddd, J = 8.4, 2.6, 1.5 Hz, 1H), 8.96 (dd, J = 4.8, 1.5 Hz, 1H), 9.46 (d, J = 2.6 Hz, 1H).
3. Results and Discussion
In continuation of our previous studies on the application of TCS in organic synthesis [45] - [47] herein we report a simple, mild and convenient regioselective nitration of different aromatic hydrocarbons using TCS and inorganic nitrate salt (Figure 1).
This combination was not reported earlier in the literature and we are currently exploring the potential using of this reagent system in further organic transformations. Our main objective was to overcome the nitration’s associated drawbacks such as acidic corrosivity, tedious work up and safety problems (preparation, storage and handling of the nitrating agent). Furthermore, the use of one-pot synthesis has attracted our interest since it is easily performed and usually offers high yield.
In order to find out suitable reaction conditions, the reaction of phenol with TCS and NaNO3 was preliminary examined in different solvents and at different molar ratios. The use of ethanol leads to exothermic reaction, low yielding and the formation of by-products. Oxygenated donor solvents (e.g., tetrahydrofuran, diethyl ethers) were also non convenient as they completely inhibited the reaction (Table 1).
The use of chloroform and dichloromethane led to minimization of the side products; however, reaction in dichloromethane affords better yield than in chloroform. We therefore decided to perform the reaction in dichloromethane and optimize the other reaction conditions. The reaction was then carried out using different molar ratios of TCS, NaNO3 with respect to phenol in dichloromethane. It is worth noting that, no reaction was observed in the absence of TCS. Furthermore, the use of equimolar amounts of reagent (TCS-NaNO3) and substrate led to large quantities recovery of the staring materials. NaNO3 concentration was therefore increased in order to improve the yield. This was found to be ineffective neither on the reaction productivity nor to the reaction selectivity (monitored by TLC). Interestingly, yields improvement and by-products minimization were observed via increasing the concentration of the TCS (monitored by TLC). After a long term of trials, good yields were obtained when the TCS molar ratio was twice the aromatic hydrocarbon and the nitrate salt i.e. aromatic substrate: nitrate salt: TCS molar ratio was 1:1:2, respectively (Table 1). Further increment of the molar ratio of TCS led to the formation of a mixture of di- and tri-nitro derivatives. The reaction was performed under the dark and at room temperature.
The typical reaction accomplishment time was up to 6 hours (monitored by TLC). This led us to consider the addition of catalyst (e.g., Lewis acids) in order to reduce the reaction time. The addition of ZnCl2 (10 mol%) is associated with rate enhancement and reduction of the reaction time (up to 2 h) (see experimental part for more details).
A reasonable mechanism for the present reaction might proceed as depicted in Figure 2. It was assumed that (O2NO)SiCl3 intermediate was generated and its further reacted with excess TCS to form nitryl chloride (NO2Cl).
It is though that the in situ formed NO2Cl is further activated via coordination with ZnCl2 to give the corres-
![]()
Figure 1. Nitration of aromatic hydrocarbons using tetrachlorosilane-sodium nitrate homogeneous system.
ponding nitronium salt 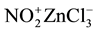 which in turn is considered to be a very reactive nitrating agent (even more powerful than the dinitrogen pentoxide) (Figure 3). The later acted as the nitronium source and ultimately reacted with aromatic hydrocarbons to give the corresponding mononitro derivatives.
which in turn is considered to be a very reactive nitrating agent (even more powerful than the dinitrogen pentoxide) (Figure 3). The later acted as the nitronium source and ultimately reacted with aromatic hydrocarbons to give the corresponding mononitro derivatives.
This pilot reaction endured a considerable diversity of functionalized aromatics (e.g., benzene, naphthalene and alkyl, alkoxy, hydroxy and acetyl substituted-benzene) (Table 2). The nitration of benzene, naphthalene, phenol, 2-methylphenol and 3-methylphenol offered high yield (60% - 92%). On the other hand, acetophenone were nitrated in low yield (40%).
The para isomer was isolated in most cases. The reason was mainly attributed to steric hindrance between the substituents and the electrophilic group, especially in the ortho-position. On the other hand, no reaction was observed in case of benzoic acid, aniline and benzaldehyde. Groups that withdraw electron density (e.g. COOH and CHO) causes strong deactivation. This may be the reason that no nitration reaction was observed in case of benzoic acid and benzaldehyde.
Despite the fact that the NH2 group is a much more powerful activating group than COOH and CHO, aniline also failed to react under these conditions and a black tar is formed. This may be attributed to the side reaction
![]()
Figure 2. Formation of nitryl chloride, the source of nitronium salt .
.
![]()
Figure 3. Activation of nitryl chloride by coordination with zinc chloride.
![]()
Table 2. Nitration of various aromatic hydrocarbons to their corresponding mono nitro derivative employing SiCl4/ZnCl2/ NaNO3 combination in dichloromethane.
a. All the reactions were performed using 1mmol of substrate, 1 mmol of NaNO3, 2 mmol of TCS and catalytic amount of ZnCl2 (10% mmol).
which might take place between the TCS and the NH2 group (e.g., oxidation). Currently, this reaction is being expanded in our lab employing different conditions (solvents and catalysts) and with wider variety of substrates. On the other hand, the strongly activating, electron donating, -OH group(s) favour aromatic nitration via the activation of the ortho-para centers. In this case, the highest electron density is located on both ortho and para positions due to resonance donating effect of the hydroxyl group.
4. Conclusion
A novel and general methodology for efficient nitration of aromatic hydrocarbons using TCS-NaNO3 binary reagent and ZnCl2 was reported. This method is easy to perform and provides moderate-high yields. In order to establish the complete picture about the durability and efficiency of this method, some points are needed to be further clarified. This includes the evidence that ZnCl2 provides a positive effect not only on the activation of NO2Cl but also on the other steps (see the mechanism). Moreover, more data with further experiments such as 29Si NMR study and MS analysis using considerably wider functionalities of aromatic, aliphatic and heterocyclic hydrocarbons need to be further investigated. We see a plenty of room for further, multi-disciplinary studies involving synthetic and mechanistic studies in order to develop convenient nitration method by applying TCS.
Acknowledgements
The authors wish to express their sincere thanks to staff members of Organic Chemistry Research Labs. at Chemistry Department, Faculty of Science, Mansoura University, Egypt for the facilities and support provided.
NOTES
*Corresponding author.