Replacement of Cereal with Low Starch Fibrous By-Products on Nutrients Utilization and Methane Emissions in Dairy Goats ()
1. Introduction
Feeding systems for dairy ruminants need to ensure high intake of energy, among other factors, to achieve maximum milk production potential. This might be accomplished by raising the dietary concentration of rapidly degraded non fibrous carbohydrates, such as starch from cereal grain. Increasing the concentration of starch in diets for dairy cows, however, can lead to undesirable ruminal fermentation, compromising the nutrient supply for production of milk and milk components. To prevent ruminal upsets and health problem, the NRC [1] recommended the partial replacement of cereal grain with low starch by-product feeds.
Increasing fibrous by-products in the diets generally results in considerably higher enteric CH4 formation compared with starch based diets [2] . The review of [3] concluded that the inclusion of fibrous by-products in the diet of ruminants would likely increase enteric CH4, particularly when inclusion was above 35% to 40% of dry matter intake. Feed ingredients provide the substrates for microbial fermentation, and differences in feed digestibility and chemical composition alter the amount of energy extracted by the microbes and the pattern of volatile fatty acids and CH4 produced [4] .
Animal diet can also have a significant impact on manure composition and subsequent gaseous losses [5] . Modifying proportions of carbohydrates in the diet may lead to different feces composition, which, in turn, will result in different green house gas emissions. Nevertheless, there is scarce information on the effect of replacing carbohydrate sources on ruminant diets over CH4 emissions from their manure [6] -[8] .
The purpose of the present investigation was to compare the effect of fed two mixed diets to dairy goats differing in the type of carbohydrate (starch vs. potentially digestible fiber) in relation to 1) energy, nitrogen (N) balance, and milk performance, and 2) enteric and manure CH4 productions.
2. Materials and Methods
2.1. Animals and feeding
The experimental procedure was approved by the Animal Use and Care Committee of the Polytechnic University of Valencia (Spain) and followed the codes of practice for animals used in experimental works proposed by the European Union [9] . Ten multiparous mature Murciano-Granadina goats of similar body weight (43.01 ± 1.7 kg BW, mean and standard deviation, respectively), 625 ± 4.7 kg in 210 days of lactation, 4th lactation, in mid lactation (106 days in milk) were randomly split into two groups. Nutrient requirements followed the recommendation of [10] and [11] for goats during lactation. Experimental design was in a cross over (2 treatments × 2 periods) and, goats were fed two different mixed rations, one group was fed a mixed ration with 364 g/kg dry matter (DM) of barley grain (high starch diet, HS) and the other diet substituted with barley by 446 g/kg DM of by-products (low starch diet, LS) in the following proportion: 271 g/kg DM soy hulls and 175 g/kg DM gluten feed. The starch levels were 21.9% and 7.0% (both on DM basis) for HS and LS diet, respectively. The goats which are fed ad libitum and half the daily ration was offered at 0800 h and half at 1600 h, respectively. Goats had free access to water. Alfalfa hay was cut into 2.5 cm pieces (Cutter SkioldSaby A/S, Kjeldgaardsvej road, DK 9300 Denmark), and the concentrate was mixed and pelleted along with the premix (Table 1). Chemical composition showed a whole mixed ration (forage and pelleted diet). Mixed rations were isoenergetic, with an average value of 19.1 MJ/kg DM for gross energy (GE), and isoproteic 18.1% (DM basis) of CP. Body weight at the beginning and end of the period was determined. The goats were milked once daily at 0700 h with a portable milking machine (Flaco, model DL-170, J. Delgado S.A., Ciudad Real, Spain).
2.2. Experimental Schedule and Measurements
Goats were fed experimental diets on pens during 10 days, then they were allocated in individual metabolism cages at thermoneutrality for another 10 days (20˚C - 23˚C). Total tract apparent digestibility, energy and N balance, and milk performance were determined for each animal during a period of 5 consecutive days (five goats
![]()
Table 1. Ingredients (g/kg DM) and chemical composition of diets (% of DM): high starch (HS) and low starch (LS) diets.
aHS, high starch diet; LS, low starch diet; DM, dry matter; bFused lard provided by VALGESS S.L., Carpesa, Valencia, Spain; cBy-pass fat of palm fatty acid distillate. Provided by Norel Animal Nutrition, Norel S.A., Spain; dPremix: provided by NACOOP S.A. España. (ppm or UI per kilogram of premix): Se, 40; I, 250; Co, 80; Cu, 3000; Fe, 6000; Zn, 23,400; Mn, 29,000; S, 60,000; Mg, 60,000; vitamin A, 2,000,000 UI; vitamin D3, 400,000; vitamin E, 2000 ppm; nicotinic acid, 10,000; choline, 20,300; eNFC, nonfibrous carbohydrate content: 100-(NDF + ash + CP + EE).
per treatment). Feed intake and refusal, and fecal, urine and milk outputs were recorded daily for each goat. Total feces collection were collected in wire-screen baskets placed under the floor of the metabolism crates, and total urine collection was collected through a funnel into plastic buckets containing 100 mL of 10% (vol/vol) H2SO4. Representative samples (20%) of diet, feces and urine were stored at −20˚C, and pooled for chemical analysis. Immediately after milking, the individual milk yield was weighted and, after mixing, a sample of 10% was put in a bottle with 20 mg of potassium dichromate as a preservative and stored at 4˚C before analyses. Ruminal fluid samples were collected by stomach tube before the morning feeding on the last day of the apparent digestibility trial. Ruminal fluid pH was immediately determined using a Model 265A portable pH meter (Orion Research Inc., Beverly, MA, USA). A 5 mL subsample of strained ruminal fluid was acidified to pH 2.0 and mixed with 50% H2SO4 and frozen until analysis for short chain fatty acids (SCFA).
Gas exchange was measured for each goat during 24 h by a head-hood designed for small ruminants.We have one head hood and it was placed in an isolate room without contact with others goats or rest of herd. The head hood dimensions were 36 cm deep × 53 cm wide × 116 cm high, giving a total internal volume of 219 L. The hood was fitted with a polycarbonate window and drawer at the front to facilitate feeding and watering. A tightly woven nylon curtain with a hole for the animal neck, which was attached to the rear panel of the hood, was tied around the animal neck with a nylon drawstring to minimize gas leakage. Fresh outdoor air was introduced into the hood via a hose connected to a box entrance. The gas outlet was across a pipe attached on top of the hood equipped with an air filter to prevent dust in the circuit. Through this pipe the gas flowing from the ventilated head hood to the open-circuit respiratory system, which monitored gaseous exchanges by each animal.
Total airflow through the system was measured by a mass flow meter (Thermal Mass Flowmeter Sensyflow VT-S, ABB, Alzenau, Germany). Air suction was by a centrifugal fan (CST60 Soler Palau Inc., Parets del Vallès, Barcelona, Spain) located at the end of the main sampling line with free escape for the air. The gas analyser (Easyflow 3020 model, ABB, Alzenau, Germany) was calibrated with reference gases. Description of the mobile open-circuit respirometry system used for these measurements was shown in [12] and [13] .
The whole system was calibrated injecting pure N2, CO2 and CH4 into the head box [14] determined gravimetrically using a precision scale. Calibration factors were calculated according to [15] . The average value for the calibration factor was 1.0056 ± 0.00158, 0.9924 ± 0.00915 and 0.9321 ± 0.0053 for O2, CO2 and CH4, respectively. The CH4 and CO2 production and O2 consumption were calculated as described [16] . An initial atmospheric air sample was collected previous to start the gas exchange measurements with animals and, the gas concentrations were used as reference for calculations.
2.3. Feces Incubation Experiment
Feces were mixed per dietary treatment and stored at 4˚C until measurement of gas emissions. Dry matter and organic matter (OM) content of HS and LS-derived feces were analyzed before and at the end of the incubation period.
Methane production was determined in a batch experiment. The experiments were performed in 280 ml bottles incubated at 38˚C, controlled by a thermostat (Selecta, Termotronic) with 3 replicates for each treatment. According to [17] , CH4 production was maximized by diluting samples (1:9 ratio) to avoid any inhibition by excessive NH3-N concentration. Nitrogen content in the samples was 2% (DM), allowing a headspace ratio equal to 1.6 (manure diluted/headspace). After filling with faecal sample, bottles were sealed with butyl rubber stoppers and the headspace was flushed with N2 gas during 1 minute.
During incubation, the volume of gas produced was calculated by measuring pressure in the headspace using a manometer (Delta Ohm HD 9220, absolute pressure meter, 0 - 2000 mbar) and a gas sample was collected in a 9 mL GC vial. After gaseous sample collection, overpressure was removed to restore the atmospheric pressure. Incubation lasted 42 days and measurements were taken in four occasions (days 14, 21, 35 and 42 since start of incubations).
The concentration of CH4 in biogas was determined by gas chromatography. An Agilent gas chromatograph, model 7890A coupled to a flame ionization detector (FID) and a HT3 Teledyne Tekmarhead-spaceautosampler (HS) was used to perform the analysis. The GC column used was an Alltech (30 m × 0.32 mm × 0 µm film thickness) capillary column and the oven temperature program was 50˚C for 3 min, then raised to 75˚C at 6˚C and held for 5 min. The FID temperature was 250˚C and helium was used as the carrier gas (24 psi).
2.4. Chemical Analysis
Feed, feed refusal and feces samples were dried in a forced air oven at 55˚C for 48 h and then ground to pass a 1 mm screen. Urine was dried by lyophilization. Chemical analyses of the diet, feed refusals and feces were conducted for DM, ash, ether extract (EE) and crude protein (CP) according to [18] . DM of diets and feces was determined by oven-drying at 102˚C ± 2˚C for 24 h and OM was determined by incineration in an electric muffle furnace at 550˚C for 6 h. EE was extracted with petroleum ether after acid hydrolysis to recover saponified fat (Soxtec System HT Tecator, Hillerød, Denmark; 1047 Hydrolyzing Unit and 1043 Extraction Unit). The acid detergent fiber (ADF) and neutral detergent fiber (NDF) were measured in an ANKOM Fiber Analyzer (A220, ANKOM Technologies, Fairport, NY, USA) according to [19] using sodium sulphite and alpha amylase. Non- fibrous carbohydrates (NFC) content of diets was calculated by difference method based on chemical analysis of individual feeds as [1] shown: NFC = 100 − NDF − ash − CP − EE. Gross energy content of the dried samples (feed, feces, urine and milk) was analyzed in an adiabatic bomb calorimeter (Gallenkamp Autobomb; Loughborough, UK). The N was analysed by the Dumas principle (TruSpec CN; LECO Corporation, St. Joseph, MI, USA). Multiplying N by a factor of 6.25 converted the results to CP. Milk composition (fat, protein, lactose, and total milk solids content) was analyzed with infrared analyzer (MilkoScan FT120 Foss Electric, Hillerød, Denmark). Starch content was determined by enzymatic method (alpha amylase obtained from Sigma-Aldrich, Steinheim, Germany) according to [20] .
Determination of ruminalshort chain fatty acids (SCFA) was based on the method described by [21] . One µl from each sample was injected in a gas chromatograph (Fisons 8000 series, Milan, Italy) equipped with a split/ splitless injector and FID detector. The separation of SCFA was made in a DB-FFAP capillary column (30 m × 0.25 mm × 0.25 µm of film thickness) J&W Scientific (USA).
2.5. Calculations
The ME intake (MEI) was calculated as the difference between GE intake and energy losses in feces, urine and CH4 (with an energy equivalent value of 39.5 kJ/l CH4, [22] ). The heat production (HP) was calculated according to [22] for O2 consumption (l/d), CO2 and CH4 production (l/d) and urine-N (Nur, g/d) as:
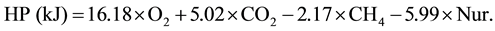
The retained energy (RE) was calculated as the difference between MEI and HP. The body tissue energy (TEbody) was the difference between RE and milk energy (Emilk). The N balance is based on the measurements on nitrogen in feed, feces, urine and milk.
2.6. Statistical Analysis
The effects of type of diet on intake, digestibility, energy and N balance as well as milk yield and enteric CH4 emissions were analyzed using the mixed model (proc MIXED) from SAS software [23] . The experiment was conducted as a crossover design: each goat received both treatments in 2 periods. The model for the dependent variables included the fixed effect of diet and period with goat as random effect. The following statistical model was used; Y = μ + D + T + goat + DxT + ε, where Y is the dependent variable, μ is the overall mean, D and T are the fixed effects of diet and period of time, DxT their interaction, goat is the random effect of goat and ε is the random error. Least square means are reported throughout and differences were considered significant at P < 0.05.
The accumulated amount of biogas and CH4emissions wascalculated by the following equations:
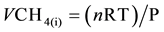 (E1)
(E1)
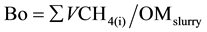 (E2)
(E2)
where n is the CH4 mol amount in headspace, R is the ideal gas constant (atm L∙K−1∙mol−1), T is the incubating temperature in Kelvin (K), Pis the pressure at the current measurement (atm), VCH4(i) is the volume of CH4 in biogas at the current measurement (L), OMslurry is the total amount of organic matter in dry weight basis in stored slurry (kg).
CH4 production data from each treatment were analyzed as repeated measurements during the study. Significant differences are expressed at P < 0.05, unless otherwise stated.
3. Results
Effect of period of time and their interaction were not significant, so there are not shown in Tables.
3.1. Feed Intake, Digestibility and Performance of Goats
Feed intake and total tract apparent digestibility of nutrients by Murciano-Granadina dairy goats are shown in Table 2. Dry matter intake was similar on both diets. Ether extract and ADF digestibility were higher (P < 0.05) with low starch diet compared with high starch diet. Total apparent tract digestibility was greater (P < 0.05) in DM, OM, CP and GE for HS diet. The CP digestibility was greater (P < 0.05) for HS diet (78%) than LS (76%). The NDF digestibility was similar in both diets. Differences were found in ADF digestibility, with greater values (P < 0.05) for LS than HS diet (41% vs. 19%, respectively). Starch was completely digested.
Milk yield was higher (P < 0.05) for HS than LS diet (2.4 vs. 2.2 kg/d, respectively), as shown in Table 3. Milk dry matter and fat content were different (P < 0.05) between the two diets with greater values for LS compared with HS (15.8 vs. 14.9% for dry matter and 6.4 and 5.5% for fat content).
Rumen fermentation parameters obtained are shown in Table 4. No differences were found for pH (average of 6.9) and, differences (P < 0.05) were found for ammonia N production (18.2 vs. 25.4 mg/dl in HS and LS diet, respectively). No differences were found for total SCFA, although differences (P < 0.05) were found in most of the SCFA studied. A significant increase of acetic, butyric and N-caproicacids in the rumen was observed in LS diet. The rest of SCFA showed differences (P < 0.05) between diets with higher values for HS diet.
3.2. Metabolic Energy Partition and Nitrogen Balance
Daily energy balance is displayed in Table 5. No significant differences were observed for GE intake (39.1 MJ/d, on average) and higher (P < 0.05) energy losses in feces (Efeces) were found for LS than HS (13.3 vs. 12.0 MJ/d, respectively). The HP was different (P < 0.05) between HS and LS treatment, with greater values for LS compared with HS (13.6 vs. 12.9 MJ/d, respectively). With regard to the rest of the energy balance, no differences were found and positive energy balance was observed between treatments.
The daily N balance is displayed in Table 6. Differences (P < 0.05) were detected for urine N in LS related with HS (26.8 vs. 21.7 g/d, respectively) and the recovered of N was higher (P < 0.05) for HS than LS (10.9 vs. 4.4 g/d, respectively).
![]()
Table 2. Daily intake, total tract digestibility and performance in lactating goats (n = 20).
HS, high starch diet; LS, low starch diet; SEM, standard error of the mean; aDMI = dry matter intake; DM = dry matter; OM = organic matter; CP = crude protein; EE = ether extract; NDF = neutral detergent fiber; ADF = acid detergent fiber; GE = gross energy.
![]()
Table 3. Milk performance in lactating goats (n = 20).
HS, high starch diet; LS, low starch diet; SEM, standard error of the mean.
![]()
Table 4. Effect of diet on ruminal pH, ammonia N (NH3 N, mg/dl) and short chain fatty acids (SCFA, mol/100mol) in lactating goats (n = 20).
HS, high starch diet; LS, low starch diet; SEM, standard error of the mean; SCFA, saturated chain fatty acids; aNH3 N = ammonianitrogen.
![]()
Table 5. Energy metabolism and nitrogen balance in lactating goats (n = 20).
HS, high starch diet; LS, low starch diet; SEM, standard error of the mean; aGEI = gross energy intake; E = energy; DEI = digestible energy intake; MEI = metabolizable energy intake; HP = heat production; TE = tissue energy.
![]()
Table 6. Energy metabolism and nitrogen balance in lactating goats (n = 20).
HS, high starch diet; LS, low starch diet; SEM, standard error of the mean; aN = nitrogen.
Table 7 shows enteric CH4 emissions from goats. The average CH4 emissions from the goat’s digestive tracts (enteric fermentation) were similar between diets and averaged at 28.5 g/goat per day. Higher values (P < 0.05) with LS than HS diet in CH4 emitted per kg of milk (13.2 vs. 11.7 g/kg, respectively) were found.
3.3. Manure CH4 Production
Goat manure characteristics before incubation are presented in Table 8. Higher OM in feces (P < 0.05) was found with LS than HS, and lower (P < 0.05) ADF was found with LS compared with HS diet (0.43 vs. 0.52 g/kg DM feces, respectively). Cumulative CH4 production of goat feces as function of time during 42 days incubation is shown in Figure 1. The CH4 production from HS (5.9 L CH4/kg OM) treatment was higher (P < 0.05) compared to LS treatment (0.28 L CH4/kg OM). The peak of CH4 production in HS treatment took place 20 days after incubation started. For LS treatment, CH4 production was negligible during the experiment.
4. Discussion
4.1. Effect of Level of Starch on Intake, Digestibility, Rumen Parameters and Performance in Dairy Goats
The higher content of barley and lower ADF content in HS diet than LS diet appeared to be the main factor responsible for the lower DM, OM and CP apparent digestibility found in LS diet. According to [1] , soy hulls and corn gluten feed are two by-products feeds that are highly digestible and are low in non-fibrous carbohydrates. Therefore, differences in digestibility depend on type and dietary proportions of different carbohydrates such as, cellulose, hemicellulose, pectin, starch, etc.
Total starch digestibility was found in both diets. Regarding fiber digestibility, lower ADF digestibility was observed for HS diet compared with LS, and it could be important regarding fecal fermentation. Non fibrous
![]()
Table 7. Enteric methane formation in lactating goats (n = 20).
HS, high starch diet; LS, low starch diet; SEM, standard error of the mean; aCH4 = methane; DMI = dry matter intake; OM = organic matter; CP = crude protein; EE = ether extract; NDF = neutral detergent fiber; ADF = acid detergent fiber; GEi = gross energy intake.
![]()
Table 8. Chemical composition of the feces previous to incubation (g/kg DM feces).
HS, high starch diet; LS, low starch diet; SEM, standard error of the mean. aOM = organic matter; CP = crude protein; EE = ether extract; NDF = neutral detergent fiber; ADF = acid detergent fiber.
![]()
Figure 1. Cumulative methane production Bo (L CH4/kg OM) from LS and HS diets in diluted goat faeces during 42 days ofincubation.
carbohydrates constitute an important energy rich fraction of ruminant diets, which consists mostly of starch, pectin, galactans and simple sugars. Non fibrous carbohydrates usually have a fast fermentation rate and stimulate the production of propionate. In our study, HS diet has greater values of both NFC (28.7% and 21.8%, for HS and LS diet, respectively) and propionic acid (21.4 and 18.0 mol/100 mol for HS and LS diet, respectively) than LS diet (higher in acetic acid). The propionic acid concentration was higher on HS than LS which indicated that goats had more precursors available for gluconeogenesis [24] , which could increase the amount of precursors available for lactose syntheses and thereby milk production. Therefore, these higher NFC and starch in HS diet seem to be the responsible of the lower ADF digestibility, minor ammonia N in the rumen, more excretion of N in urine and lower fat content in milk.
Milk yield was higher (P < 0.05) for HS than LS diet (2.4 and 2.2 kg/d, respectively), as is shown in Table 3. Milk fat content was greater for LS than HS (6.4 and 5.5%, respectively) as it had greater fiber and fat content, superior ADF digestibility and, higher acetic acid production than HS. The depression in milk fat upon feeding starch rich diets has been explained by a shift from a high availability of fat precursors to glucose and by a shift from lipogenesis to gluconeogenesis [24] . In our study, no differences were found for milk protein and lactose contents between diets (3.9% and 4.7% respectively, on average).
4.2. Effect of Level of Starch on Metabolic Energy Utilization and Nitrogen Balance of the Goats
The average GE intake was 39.1 MJ/d. The MEI was the same for the two diets (23.9 MJ/d, on average) and the HP was different between diets, with greater value for the diet higher in fiber (13.6 vs. 12.9 MJ/din LS and HS, respectively). Lipogenic nutrients, which increase milk fat yield [24] , increase the partitioning of ME into milk and consequently decrease the partitioning of ME into body reserves. The Emilk content was the same for the two diets (9.0 MJ/d, on average). We did not find differences between diets for tissue energy deposition (1.6 MJ/d, on average) and, greater efficiency of ME use for lactation (38%) than retention (7%) was observed in both diets.
More energy losses in feces were found for LS than HS (13.3 vs. 12.0 MJ/d, respectively), probably due to goat excreted more feces on LS than HS (Table 8). The Eurine was 1 MJ/d on average for the two diets and, higher excretion of N in urine was found for LS diet. These observations are in agreement with the diet rich in fibrous ingredients (LS) alongside the larger ammonia N found (Table 4). Goats fed LS diet contain greater fibrous by-products and superior ammonia N production (P < 0.05) than HS diet (25.4 vs. 18.2 mg/dl, respectively). This difference was probably linked to the lower N expenditure by ruminal bacteria to synthesize microbial protein in LS diet [25] . The Nurine losses were greater in LS compared with HS (26.8 vs. 21.7 g/d, respectively) likely due to poor efficiency for protein use (greater values of ammonia N on ruminal liquor in LS than HS, Table 4). When carbohydrate availability increases, ammonia production decreases because of a direct incorporation of ammonia N into microbial protein. Ruminal ammonia N not utilized for microbial protein synthesis is excreted in urine [26] . Diet higher in starch seems to increase the amount of fat synthesis in the body [27] and therefore energy retention. In our study no differences in energy retention were found between diets.
4.3. Effect of Starch and Potentially Digestible Fiber on Enteric Methane Emissions and Manure CH4 Production
Energy losses in CH4 were not significantly different among treatments, with an average value of 1.6 MJ/d (Ta- ble 5). The CH4 values obtained by other authors for lactating goats are variable, so [28] found values that range from 1.2 to 1.9 MJ/d, both with diets based on pelleted alfalfa hay and barley. Average values of 2.6 and 2.3 MJ/d lost as enteric CH4 for forage and non forage diets, respectively, were found by [29] .
The average CH4 emissions from the goat’s digestive tracts (enteric fermentation) was similar between diets; averaged at 28.5 g/goat per day and Ym (methane conversion factor) at 4.1 (Table 7). In our study the feed intake during the digestibility was the same that during CH4 emissions measurements. It is often claimed that forage based diets generally results in considerably higher enteric CH4 formation than mixed or concentrated based diets [30] . But [3] indicate that inclusion of fibrous by-products in the diet of ruminant will increase enteric CH4 particularly when inclusion is above 35% to 40% of DMI, and in our study was lower. The type of fiber (highly fermentable, such as the by-product used) is very different from that is usually associated to CH4 production (fiber from forages), lack of synchronization of carbohydrates and protein in LS diet (significant higher values on ruminal liquor ammonia N) and the fat level in LS (1% point higher than HS diet) were the responsible of the absence of effect of LS diet on CH4 enteric emissions. High acetic acid productions are accompanied with high levels of available hydrogen and hence with high methane production. However, soluble sugars modify the process. Fiber by-products contain more soluble sugars than roughage. Greater proportion of butyric acid was observed in LS compared with HS, because fiber quality of by-products could be favour fermentation processes to butyric acid [31] .
The DM and OM content in goat feces before incubation was slightly higher than data reported in previous studies for other ruminants [32] . The highest CH4 production in HS treatment took place 20 days after incubation started. For LS treatment, CH4 production was negligible during the experiment. There were significantly higher amount of carbohydrates available for fermentation in excreta from HS than from LS, which could have given as result higher amounts of CH4 production from HS diet, as shown in Figure 1. In brief, in our study starch was 100% digested so there was not starch in the feces; however, the digestibility of ADF with HS diet was low compared with LS diet, indicating higher hemicellulose in feces (see Table 2).
Reports in the literature on the effects of different types of diets on CH4 emissions from the goat exhaled air or manure are very scarce. Methane emissions from the feces increased shortly after the start of incubation and reached its peak between weeks 2 and 3. Several authors, [4] and [17] , observed that with cattle manure the plateau phase during batch digestion was reached later, between weeks 6 and 9. In this study, HS diet with mainly barley as concentrate had low digestibility of ADF and greater residue of ADF in feces. Consequently, manure derived maximum potential yield (Bo) was higher in HS diet (5.9 L CH4/kg OM) than LS diet (0.28 L CH4/kg OM). The review of [33] denotes that feed ingredients provide the substrates for microbial fermentation, and differences in feed digestibility and chemical composition alter the amount of CH4 production.
5. Conclusion
Comparison of starch diet with potentially digestible fiber ingredients was evaluated. Positive energy and N balance were obtained in all goats, independent of the treatment. Lower milk production was found in LS compared with HS (2.2 vs. 2.4 kg/d, respectively). Greater fat content in milk was found in the low starch diet (6.4%) compared with the higher (5.5%), because low starch diet replaced barley with fibrous by-products. Both diets showed identical Ym values (4.1% on average), so the type of fiber used to reduce the level of starch and the higher level of fat added to diet LS (1 point higher than HS) were likely responsible for the lack of effect on CH4 enteric emissions. The CH4 production from manure derived from the rich starch diet was 15 times higher than from the low starch diet after 42 days’ incubation, due to greater amount of hemicelluloses in HS feces. Goats at mid lactation could utilize fibrous by-product as cereal replacement, without detrimental effects on energy metabolism, milk performance and CH4 emissions.
Acknowledgements
This study was supported by INIA Project, Spain (ref. RTA2011-00107-C02).
NOTES
*Corresponding author.