Allelopathic Potential of Wheat on Sourgrass Resistant to Glyphosate ()
1. Introduction
Recent genetically modified organism (GMO) technologies have aimed to employ herbicide resistance to soybean cultivars, and have drastically reduced infestation of weed species in most fields with such technologies. However, lack of government regulation and farmers’ mismanagement of such technologies led to selection of weed species tolerant or resistant to herbicides applied with these technologies [1] . The glyphosate-tolerant soybean was widely used in Brazil, and currently Conyza bonariensis (hairy fleabane), C. canadensis (horseweed), C. sumatrensis (sumatran fleabane), Digitaria insularis (sourgrass), and Lolium multiflorum (italian ryegrass) are resistant to glyphosate in Brazil, due to its extensive use in Roundup Ready® soybean [2] . Species of Conyza (fleabane, horseweed) and sourgrass are considered the most troublesome weeds in Brazilian Roundup Ready® soybean cropping systems [3] .
One of the main management practices which contribute to the reduction in weeds infestation is the continuous maintenance of straw on soil [4] [5] , which among other impacts, limits weeds access to light. In addition, some plant species produce chemicals which are accumulated in soil, inhibiting germination and/or growth of other plant species. This phenomenon is called allelopathy [6] .
It is widely known in Brazilian agriculture the allelopathic effect of wheat on hairy fleanabe, horseweed and sumatran fleabane [7] [8] . Paula et al. [7] reported that, at the pre-planting of soybeans, hairy fleabane infestation was reduced from 172 plants∙m−2 to 43 plants∙m−2 only due to wheat cultivation in the preceding winter. Furthermore, infestation was reduced to 15 plants∙m−2 if iodosulfuron-methyl was used in wheat. Lamego et al. [8] highlight that in addition to a winter species efficient in reducing Conyza establishment, a good burndown prior to planting soybean is essential to reduce problems post-emergence.
We took this effect of wheat on Conyza spp. as a background for further studies. Although the effect of wheat on Conyza spp. is well known and widely used by farmers from Conyza-infested regions, there is no information about its effects in sourgrass, whose infestation is becoming widespread [9] . Considering our observations in soybean-wheat fields regarding weed infestation and population change, we hypothesize that wheat presents little to no efficiency in suppressing sourgrass compared with the level that it affects species of Conyza. Thus, we aimed with this study to assay the allelopathic potential of wheat genotypes in inhibiting germination and initial growth of sourgrass (Digitaria insularis) resistant to the herbicide glyphosate.
2. Materials and Methods
Two experiments were installed under greenhouse conditions. The first experiment aimed to screen the potential of wheat genotypes in inhibiting emergence and initial growth of test plants, and the second to assess the potential of those wheat lineages which performed better at the first experiment, in inhibiting the emergence and initial growth of sourgrass (Digitaria insularis) biotypes resistant to the herbicide glyphosate [10] .
Experiment 1
Thirty two cultivars and lineages of wheat with distinct genetic origins from the breeding program of Embrapa Wheat (Passo Fundo-RS) at the stage of pre-selection for being launched as Cultivars were used at the first study. The study was installed under greenhouse in completely randomized design with factorial scheme 32 × 6 × 2, with five replications. Factor A comprised the 32 wheat lineages (numbered 01 - 32), factor B six doses of previously prepared wheat extract, and factor C the two plant species used as indicators of inhibiting ability.
Experimental plots were composed by plastic pots with capacity for 2 L of soil, which were filled with 1.6 kg of Oxisol with 60% clay and 2.6% organic matter, where six seeds of tomato or cucumber, according to the treatment, were planted 1 cm depth.
Wheat plants were collected at heading stage (10.1), and extracts were prepared by taking 250 g of leaves from each wheat genotype, which were put into an industrial mixer grinder plus one liter of distilled cool water for processing for three minutes at full power. The mixture was passed through a fine-mesh strainer for residue removal, and 750 mL of the resulting solution was divided into six doses: 0, 50, 100, 150, 200 and 250 mL. These doses were completed to 500 mL with distilled cool water being kept into refrigerator at 2˚C ± 0.5˚C until all samples were processed.
Doses of extracts were applied just once, on plots planted at the same day with tomato or cucumber, according to the treatment. Application was accomplished by using the extract doses obtained in laboratory, completed to 500mL with distilled water, being applied on soil surface by using a watering can, and for 14 days from planting and extract application (DAA), plots were kept wet with distilled water and plant emergence was counted from 2 to 14 DAA. Emergence speed, fresh and dry mass of cucumber and tomato plants were also evaluated and considered to infer about the differential ability of each wheat genotype in inhibiting plant emergence and vigor. Due to the huge amount of data, however, for experiment 1 only emergence speed is presented for all genotypes; plant height, fresh and dry mass were considered for selecting genotypes for the second experiment, but were not shown.
Data residuals (errors) were subjected to analysis of normality and homogeneity of variances, respectively by Shapiro-Wilk and Bartlett tests, and transformed when needed, being submitted to analysis of variance by the F-test at 5% probability. Emergence speed curve was presented based on moving averages. All analyses were run and graphs drawn into the statistical environment “R” [11] .
Experiment 2
For the second experiment, the five wheat genotypes most efficient in inhibiting the indicator species (tomato and cucumber) were chosen. The study was also conducted under greenhouse, in completely randomized design under factorial scheme 5 × 6, with same plot size, replication number, soil amount and extract processing method used in experiment 1. Factor A represented the five wheat genotypes previously selected, and factor B the doses of wheat extract.
Due to the small size of sourgrass seeds, seed samples were weighted, being exactly 1.45 g of seeds planted per plot at 0.7 - 1 cm depth. Extract samples were then applied on plots according to the previously described, at the same day of planting, and plant emergence was counted for 20 DAA. At the end of this period, height of 10 plants per plot was evaluated and plants were cut at soil level for fresh and dry mass determination, being also obtained the water content (WC) by the following formula:
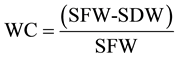 (1)
(1)
where WC = water content (%); SFW = shoot fresh weight; SDW = shoot dry weight.
Data residuals (errors) were subjected to analysis of normality and homogeneity of variances respectively by Shapiro-Wilk and Bartlett tests, and transformed when needed, being submitted to analysis of variance by the F-test at 5% probability. When treatment effect was significant, regression analysis was used for quantitative data. All analyses were run into the statistical environment “R” [11] .
3. Results and Discussion
Experiment 1
Cucumber [12] and tomato [13] are known to be sensitive to a range of compounds with allelopathic activity, being thus widely used as test plants in allelopathic bioassays. Ells and McSay [12] reports that the inhibitory effect of plants on cucumber may be assessed either by planting its seeds on the medium treated with the compound to be studied or by transplanting pre-germinated cucumber seeds to the medium. We opted for planting seeds on the medium that would receive the treatment. Shayghan and Sedghi [14] also report that bioassays with indicative plants as tomatoes are essential to characterize the potential of a given species in inhibiting growth of weed species.
Regarding the effect of the distinct genotypes on cucumber and tomato (Figure 1), there was significant difference among them in the emergence behavior of cucumber compared to tomato; for cucumber the initial emergence curve was similar for most treatments, but in contrast, the final percentage of emergence was distinct; from the six seeds planted in each plot, from 1 to 5 were emerged at 14 DAA depending on wheat genotype. Cucumber helped to discriminate genotypes according to the final percentage of emergence in detriment of emergence curve behavior (Figure 1).
For tomato an opposite behavior is observed; while the final percentage of emergence varies from 4 to 6 plants per plot, the emergence curves had distinct behavior. For wheat genotype # 06, for example, 4 plants (83%) were emerged two days after planting while for lineage # 22 this number of plants was found emerged only 14 DAA (Figure 1).
Wu et al. [15] report that the allelopathic effect of wheat on ryegrass (Lolium rigidum) was positively correlated with total phenolic acids content, and Lam et al. [16] report that hydroxamic acids are also involved in wheat allelopathic ability. Thus, although not possible in this study, the assessment of phenolic and hydroxamic contents would help selecting those genotypes with superior contents of allelopathically effective compounds in
CUCUMBER TOMATO
![]()
![]()
Figure 1. Emergence curves (y axis) of cucumber and tomato as a function of wheat genotype (1 to 32) and days after planting (x axis), following application of the full dose of the extract (250 mL plot−1). Emergence speed tendency is presented as moving averages. Embrapa Agropecuária Oeste, Dourados, MS, Brazil, 2014.
breeding programs aiming to a possible improvement for cultural weed inhibition in cropping systems [17] . Genotypes # 03, 05, 09, 20 and 26 were the ones which promoted most pronounced inhibitory effect for most of the variables (Figure 1), being thus selected for the second experiment.
Experiment 2
Both genotype and dose of extract presented distinct effects on sourgrass emergence (Figure 2). Genotype # 03 did not affect at all plant emergence, being all doses of extract similar to the check; plants started emerging 5 DAA, being the emergence stabilized by 18 DAA with about 15 plants per plot (Figure 2). This genotype corresponds to the cv. BRS Guamirim, commercially available for farmers. We hypothesize that areas planted with this wheat cultivar may present high sourgrass infestation supposing this species is present at the soil seed bank.
Genotype # 05 affected differently sourgrass emergence as wheat extract dose was increased (Figure 2). From zero to 150 mL plot−1, no differences were observed in sourgrass emergence; when 200 or 250 mL were applied per plot, emergence speed of sourgrass was reduced in about 56% 15 DAA, and in 47% 18 DAA. Genotype # 05 corresponds to the commercially available cultivar BRS 276, which similarly to genotype # 03 is a short‑cycle variety (54 - 68 days from emergence to heading) widely used in Southern Brazil [18] .
Genotypes # 09 and # 20 were similar in behavior, where only the smaller extract dose (50 mL plot−1) was similar to the check, being the other doses similar among them. Compared to the first group, doses from 100 mL plot−1 on, for both genotypes, reduced in about 33% the final sourgrass emergence as well as emergence speed,
which can be inferred by comparing the growing divergence between curves of both groups under the same genotype as days passed (Figure 2). Genotypes # 09 and # 20 corresponds respectively to lineages IPF 100010 and PF 101009, which are not yet commercially available.
Genotype # 26, which corresponds to lineage PF 101014 also not yet commercially available, affected sourgrass emergence at any dose compared to the check (Figure 2). From 11 DAA, emergence under application of any dose of extract reached 10 plants per plot, while at the check about 15 plants per plot were observed.
Genotypes # 05 and # 26 were the most consistent ones regarding suppressive effect on sourgrass emergence, since for the former larger differences were observed between the check and higher doses tested, and the latter was efficient at any dose of extract compared to check (Figure 2). In general terms, it can be noted that wheat seems not to be effective in inhibiting sourgrass emergence since the doses tested―0, 50, 100, 150, 200 and 250 mL plot−1 correspond roughly to 0, 7.5, 15, 22.5 and 30 t∙ha−1 of dry mass, considering all regarded dilutions and transformations used at Material and Methods. The amount of straw left on soil after wheat cultivation, under field conditions, traditionally averages about 5 t∙ha−1 [19] .
The fresh and dry mass, as well as water content of sourgrass seedlings 20 DAA, is shown in Figure 3. Although graphically one is impelled to recognize differences among genotypes due to the very strict scale range presented at Y-axis, analysis of variance by the F-test showed no difference among genotypes for fresh and dry mass and water content; there was difference, however, for dose of extract.
This significance for extract leads to an uncommon result; wheat extracts applied on soil previously planted with sourgrass, although with some inhibitory effect on germination speed and total germination (Figure 2), seemed to stimulate sourgrass seedling growth after emergence as dose was increased (Figure 3). This stimulation is not related to differential soil moisture since all plots received the same amount of water at every irrigation, even at planting where check plots were irrigated with 500 mL of distilled water while the other treatments received the same volume of solution (extract concentration varied according to Material & Methods).
This behavior should be confirmed in a further study, but results were consistent both statistically as well as visually?taller and most vigorous sourgrass seedlings were reported by all evaluators, for most genotypes, as extract dose was increased. We have four hypotheses: 1) wheat suppression on sourgrass is specific for germina-
![]()
![]()
![]()
Figure 3. Fresh and dry mass and water content of sourgrass plants 20 days after emergence as a function of wheat genotype and dose of extract under controlled environment. Embrapa Agropecuária Oeste, Dourados, MS, 2012.
tion; 2) wheat/sourgrass type of interference, positive, negative or neutral, is dependent upon growth and development stages; 3) competitive aspects not documented may be affecting our results; 4) a process of allelomediation―changes to the physical or biological environment caused by wheat which in turn favors sourgrass―may be occurring. There are currently no scientific reports about allelopathic or allelomediated effects of sourgrass on other plant species [20] , but the opposite may be true. This discussion is partially out of the scope of this study, but this behavior was registered and will be further investigated.
The use of allelopathy as a less impacting weed management tool is gathering attention from the public due to its ecological and environmental appeal, but results from controlled environment studies often fail to correlate to field results, where the suppressive ability is often lower than the observed under greenhouse or laboratory trials [21] . Physical, chemical and biological soil processes may result in detoxification or inactivation of compounds with allelopathic activity, which would pose serious limitations to the results, mainly for those experiments carried out in absence of soil.
Wheat is recognized as potential inhibitor of several weed species, among them Conyza spp. [7] [8] , several other weeds and crops as rice, barley, rye and cotton, among others [16] . Based on our results we believe wheat unfortunately has little effect on sourgrass; it can reduce plant emergence at some extent, but plants which are able to germinate apparently present most pronounced initial growth compared to those grown in absence of wheat. Thus, in the cropping systems of Central Brazil, other crops should be tested aiming to help reducing sourgrass biotypes resistant to the herbicide glyphosate, just as wheat is suppressive for Conyza-infested areas.
4. Conclusion
Sourgrass (Digitaria insularis) germination is affected by wheat extracts, but wheat genotypes differ in terms of their ability in inhibiting sourgrass germination; initial seedling growth of sourgrass seems not to be affected by wheat extracts; overall, wheat seems to present little inhibitory effect on sourgrass.