Aceclofenac-Soluplus® Nanocomposites for Increased Bioavailability ()
1. Introduction
Though the oral administration is the most convenient route of delivering drugs, it has several concerns associated with bioavailability due to the complicated gastrointestinal (GI) tract physiology, first-pass metabolism and biotransformation. More often than not, low aqueous solubility in the GI conditions is the limiting factor to the bioavailability of many drugs. Approximately 40% of potential new chemical entities identified by pharmaceutical companies are poorly soluble in water, which greatly restricts their further clinical translations. Although several advanced strategies and formulations such as cyclodextrin complexes [1] conjugation to dendrimers [2] , salt formation of ionisable drugs [3] , and the use of solid dispersions [4] , formulations that can enhance the drug’s bioavailability are of great demand to the pharmaceutical world . Even as the existing techniques are progressively altered to tackle this issue, the future appears still brighter for novel technologies developed to synthesize ultrafine particles in nano-scale regime. Several nanotechnology based formulations such as nano-crystals, solid lipid nanoparticles (SLN), nano-emulsion, polymeric nanoparticles, liposomes and polymeric self-assemblies have been employed to enhance drug solubilization. Techniques such as media milling and high pressure/shear homogenization have been used for producing nanoparticles [5] . Nanosized polymeric particles have also emerged as a pragmatic approach for the efficient formulation of hydrophobic drugs. The major characteristic of these systems is the rapid dissolution rate which enhances bioavailability after oral administration.
Aceclofenac is a new generational Non-Steroidal Anti-Inflammatory Drug (NSAID), and is considered a better alternative to the diclofenac and other NSAIDs, as it ensures more gastrointestinal safety than most NSAIDs [6] . However, the bioavailability of the drug remains limited due to low aqueous solubility (0.058µg/mL) and poor dissolution characteristics [7] . Hence, improving its dissolution characteristics is of prime significance in order to establish its optimal therapeutic efficacy. Several efforts have been made to improve the dissolution characteristics of aceclofenac [7] -[10] . With an increasing trend to seek nanotechnology based solutions to medical related problems, the use of nanoparticles for improving the bioavailability of poorly soluble drugs is, now, widely being explored [11] [12] .
We, here, report the use of the novel polymer Soluplus® to manufacture nanoformulations of aceclofenac by high shear homogenization via ultrasonication followed by drying by lyophillization.
Soluplus® is an amphiphilic graft co-polymer (polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol), specially manufactured by BASF for formulating poorly soluble drugs. Due to its bi-functional nature, it is expected to act as an excellent matrix to dissolve the drugs in aqueous medium. Soluplus is a novel polymer specially designed for the fourth generation solid solutions towards dissolution enhancement [13] . Soluplus can increase solubility and bioavailability of poorly soluble drugs. It is ideal for hot melt extrusion with excellent extrudability and easy processing [14] . The main objective of this work is to investigate the feasibility of synthesizing aceclofenac nanoparticles in Soluplus® matrix using the single emulsion technique in an effort to establish the enhancement of wetting characteristics, while decreasing the agglomeration of aceclofenac nanoparticles, and to evaluate their drug content and loading efficiency, IR and XRD spectral characteristics, morphology and investigate their solubility and dissolution rate.
2. Materials and Methods
2.1. Materials
Aceclofenac or 2-[2-[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxyacetic acid was obtained as a gift sample from SKN Organics, Pondicherry. Soluplus® graft co-polymer (polyvinyl caprolactam-polyvinyl acetate-poly- ethylene glycol) was acquired from BASF, Mumbai respectively. All the other chemicals used were of analytical grade.
2.2. Preparation of Aceclofenac Nanomatrix Systems
Nanoemulsions of aceclofenac in Soluplus® were synthesized using the single emulsion technique. The drug and the polymer were dissolved in dicholoromethane (DCM) in ratios of 1:1, 1:2 and 1:5 to form the organic phase and 20 mL of distilled water with 0.05% Tween 80® surfactant formed the aqueous phase. Using a syringe pump, the organic phase was slowly dropped into the aqueous phase (flow rate; 0.3 mL per minute), while continuous probe ultrasonication in an ice bath was used for effective homogenization of the two phases. The mixture was then magnetically stirred continuously for 24 hours for the removal of DCM and successively lyophilized (Mini Lyodel, India) at −40˚C for about 12 hours and gently ground to obtain free flowing powders. The powders were then sieved though mesh of 200 µm sieve size and preserved under desiccation. The samples were labeled as NP AS 1, NP AS 2 and NP AS 3 to denote the nanoparticles of drug: polymer 1:1, 1:2 and 1:5 respectively.
3. Physico-Chemical Characterizations
3.1. Fourier Transform Infrared (IR) Spectroscopy
FTIR spectra were obtained using an FTIR Spectrophotometer (Spectrum FTIR (Scimadzu, IRAffinity-1)).
The spectrum was recorded in the range of 4000 - 400 cm−1. The procedure consisted of dispersing a sample in KBr followed by gentle mixing. The spectrum was scanned at a resolution of 0.15 cm−1 and scan speed was 20 scan/s.
3.2. Differential Scanning Calorimetry (DSC)
Differential Scanning Calorimeter (TGA DSC 1, Mettler Toledo) was used to characterize the thermal behavior of the samples. The samples were heated at a rate of 10˚C∙min−1 from ambient temperature to the melting point.
3.3. X-Ray Diffraction (XRD)
XRD patterns were recorded using PANalytical X’pert Pro MPD diffractometer, with the following settings: Cu Kα radiation with wavelength 1.54 Ǻ, voltage = 45 kV, current = 40 mA. Measurements were made in the 2θ range of 10˚ to 80˚.
3.4. Field Emission Scanning Electron Microscopy (FESEM)
Double sided carbon tapes were fixed on an aluminum stub. The powders were sprinkled onto the tape and gold sputtered for 10 mins. The aluminum stub was placed in the vacuum chamber of a scanning electron microscope (Carl Zeiss SMT-Super Ultra Model Gemini Ultra 55) operated at 4 kV. The particles were observed for surface characteristics.
3.5. Phase Solubility
Phase solubility of aceclofenac in presence of Soluplus was determined by the method established by Higuchi and Connors [7] . Briefly, an excess amount of the drug was introduced into a 25 ml flask with various concentrations of the polymer (0.5%, 1%, 1.5%, 2%, 2.5%, 3% and 4%w/v) in 20 mL distilled water.
The flasks were suitably sealed and shaken (100 agitations/min) in orbital shaking incubator for 24 h at 37˚C. The sealed flask where equilibrium is achieved after 24 hours at 37˚C in incubator, 5 ml of supernatant was withdrawn and filtered. The filtrates were analyzed using a UV-visible spectrophotometer at 273 nm after suitable dilution. The measurements were performed six times.
The ΔG˚tr value provides information about whether the treatment is favourable or unfavourable for drug solubilization in an aqueous medium [7] . Negative Gibbs-free energy values indicate improved dissolution [15] . The ΔG˚tr values of aceclofenac were calculated using the following equation:
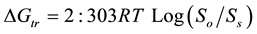
where So/Ss, is the ratio of the molar solubility of aceclofenac before and after treatment. The value of gas constant (R) is 8.31 J∙K−1∙mol−1 and T is temperature in degree kelvin.
3.6. In Vitro Dissolution Testing
The in vitro dissolution analyses were performed using a USP type II dissolution testing paddle apparatus (DBK Dissolution Tester, Mumbai, India). A known amount of sample (equivalent to 50 mg of aceclofenac) was introduced into the glass jar of the USP type II paddle apparatus containing 900 mL of simulated intestinal fluid. This was stirred at 70 rpm for 2 hours. After predetermined regular intervals, 3 mL aliquots of the sample were withdrawn, filtered, suitably diluted and the concentrations of the withdrawn solutions were determined using a UV spectrophotometer (Schimadzu, UV 2450). To maintain a constant volume during dissolution, 3 mL of solution was replaced into the glass jar after every withdrawal. Corrections for this dilution were made during the calculations. The percentage of the drug dissolved was calculated and plotted versus time. These studies were carried out three times.
4. Results and Discussion
4.1. Phase Solubility
The influence of Soluplus® on solubility of aceclofenac in distilled water at 37˚C is presented in Table 1. At 7% and 10% w/v concentrations of the polymer, the solubility of aceclofenac was increased by 4.9 times. The enhancement in solubility could result due to the amphiphilic nature of the polymer as well as surface adsorption of drug on the polymer. The values of Gibbs-free energy (ΔG˚tr) associated with the aqueous solubility of aceclofenac in the presence of Soluplus® are presented in Table 1. The ΔG˚tr values were negative at concentrations of the polymers beyond 7%, which reflect the spontaneous nature of the aceclofenac solubilization at that particular concentration of Soluplus. Also, the values decreased with increasing concentrations of polymer beyond 7%, thereby demonstrating that reaction became more favourable as the concentration of polymer was increased. This shows that Soluplus® is an ideal solubilizer for aceclofenac at concentrations beyond this percentage.
4.2. FTIR Analysis
As evident in Figure 1, the spectrum of aceclofenac showed characteristic bands at 3319.3 cm−1 (N-H stretching), 2970.2 and 2935.5 cm−1 (O-H stretching), 1716.5 cm−1 (C O stretching), 1589.2 cm−1 (skeleton vibration of aromatic C C stretching), 1506.3 cm−1 (in plane bending for N-H), 1380 cm−1 (O-H in plane bending), 1280.6 cm−1 (C-N aromatic amine), 944 cm−1 (O-H out plane bending) and 746 cm−1 (out plane bending for N-H). FTIR spectra of Soluplus® exhibit characteristic peaks at 2924 cm−1 (aliphatic-CH stretching), 1730 and 1632 cm−1 (C=O stretching). In the nanodispersions, the N-H stretching peak of aceclofenac shifted towards lower frequency 3424 cm−1 with increasing polymer ratio and completely disappeared in NPAS 3. The reason for this observation might be the consequence of hydrogen bonding between -COOH of aceclofenac and carbonyl oxygen of Soluplus®. The presence of hydrogen bonding between the drug and polymer is indicative of the stability of the formulations because the rate of crystallization of the particles depends on the molecular mobility in the dispersed phase [16] .
4.3. DSC Analysis
Figure 2 shows the thermal analysis curves for the drug, polymer and the naoformulations. Pure aceclofenac showed a distinct melting endotherm at 154˚C. The polymer Soluplus® showed a broad glass transition (Tg) endotherm at 79˚C. The nanocomposites in all the drug-polymer ratios were devoid of the drug melting endotherm and showed decreased enthalpies of melting in the nanocomposites indicating the absence of crystalline drug moieties in the matrix of the polymer. The Tg of the polymer was observed to reduce in the presence of the drug. This could be because the drug-polymer interactions substitute the polymer-polymer interactions in the nanocomposites, thus lowering the Tg. Enhanced solubility and faster dissolution rates of the drug are known to result
![]()
Table 1. Phase solubility of aceclofenac with various ratios of Soluplus®.
from the transformation of the stable crystalline state of the drug to the high disorder and high energy semi- amorphous or complete amorphous state in the formulations.
4.4. XRD Analysis
From Figure 3, it can be noted that, intense crystalline peaks of aceclofenac occurred at the diffraction angles 14.4, 17.7, 18.0, 18.6 and 24.6, while the polymer Soluplus® is an amorphous powder having no indication of crystalline structure. The nanocomposite powders were all x-ray amorphous with no crystalline peaks of the drugs at all. This is in coherence with the thermal analysis plots. It can be thus expected that aceclofenac is present as amorphous dispersions in the matrix of Soluplus®.
4.5. FESEM Imaging
The FESEM images (Figure 4) of the nanoparticulate system showed a beaded network of Soluplus® and aceclofenac composites. The absence of distinct drug crystals in the SEM images indicate that no crystallization occurred during the freeze drying process and that the drug is present as an amorphous solid or as low crystalline
![]()
Figure 1. FTIR spectra for the pure drug and the nanoparticles of the drug with various Soluplus ratios.
![]()
Figure 2. DSC thermal curves for the pure drug and the nanoparticles of aceclofenac with various Soluplus ratios.
![]()
Figure 3. XRD spectra for the pure drug and the nanoparticles of the drug with various Soluplus ratios.
![]()
Figure 4. FESEM images of the formulation of aceclofenac and Soluplus® nanoparticles of NP AS 3 formulation at various magnifications.
moieties in the formulations. The loss of crystallinity was also confirmed by DSC and XRD analyses.
4.6. Dissolution Analysis
The dissolution profiles of the pure drug and the various nanoformulations are given in Figure 5. Pure aceclofenac has a saturating dissolution profile at 40% even at the end of 2 hours. Clearly, it can be seen from the figure that dissolution profiles of the nanoformulations at all drug-polymer ratios are much higher than that of the pure drug. This could be attributed to the dispersion of the drug as highly amorphous particles in the matrix of Soluplus® and increased wettability on account of the hydrophillicity of the polymer.
The nanoformulations NPAS 1 and NPAS 2 showed similar dissolution profiles reaching 70.1% dissolution achieved in a span of 10 minutes and saturating at 65.3% release by the end of 2 hours. The formulation NPAS 3, with the drug to polymer ratio of 1:5 showed maximum dissolution of 95.2% in 5 minutes, though the dissolution profile saturates at 86.6% by the end of 2 hours. Even with the physical mixture of aceclofenac and Soluplus® a marginal enhancement in the solubility with increase in polymeric content could be seen. This could be due to surface activity and its solubilizing wetting characteristics arising from the amphiphillicity of the polymer [17] .
The possible mechanism of solubilization of the aceclofenac nanoparticles could be explained in the following manner. The drug nanoparticles were well dispersed in the polymer matrix as a result of the proper miscibility of the drug with the caprolactam (hydrophobic) part of the polymer. As the drug-polymer composite came in contact with water, the ethylene glycol (hydrophilic) part of the polymer hydrated rapidly into solution, solubilizing the dispersed drug nanoparticles as well. Also, the reduction in particle size increases the surface/volume ratio and the surface interactions [18] , thus resulting in proper drug-polymer miscibility and enhancement of the dissolution rate and solubility and consequently the bioavailability of the drug [19] .
5. Conclusion
The present study shows the utility of a novel amphiphillic carrier Soluplus® in synthesis of aceclofenac nanoparticles to enhance its solubility and dissolution. The single emulsion method is adopted to convert crystalline form of drug to a more energetic amorphous form in stable nanoparticles. The results indicate that Soluplus enhances the dissolution of aceclofenac and also provides physical stability by preventing reversion of drug from crystalline state to amorphous state after lyophillization. The drug release from the nanoparticles is far superior to that of pure aceclofenac as well as the physical mixtures. This can be due to particle size reduction and the loss of crystallinity of the drug as nanoparticles, and also the increased wettability of the drug in the matrix of the amphiphilic polymer. This approach can be further extended for dissolution enhancement of other poorly soluble BCS class II drugs.
![]()
Figure 5. Dissolution analysis of the pure drug, physical mixtures of drug and polymer and the nanoparticles with various ratios of the polymer.
Acknowledgements
The authors are grateful to their Chancellor, Bhagwan Sri Sathya Sai Baba for His inspiration and guidance. They would also like to thank BASF, Mumbai and SKN Organics, Pondicherry, for providing them with the polymer and the drug respectively. The author, Sandeep Patnaik, would like to thank the University Grants Commission for the Basic Science Research fellowship.