1. Introduction
Nitrogen fertilizer is the most widely used fertilizer (300 - 350 kg/Ha) on the corn plant, but because it has mobile characterization, it becomes the lowest utilization (only 40% - 50%) compared to other nutrient elements [1] . Approximately-estimated 50% of the fertilizer N in wet tropical areas will be lost [2] . Although corn plants can absorb the N in the form of  and
and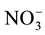 , but
, but 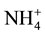 form is more efficient because it requires lower photosynthesis energy for reduced in NH3 i.e. 5ATP per
form is more efficient because it requires lower photosynthesis energy for reduced in NH3 i.e. 5ATP per 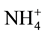 molecule, where as
molecule, where as 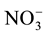 requires 20ATP per molecule [3] . Inhibition of nitrification can increase the efficiency usage of photosynthesis so it can increase the dry weight of the plant. Proven that N uptake in wheat would increase by 35%, if 25% of its fertilizer N was in the form of
requires 20ATP per molecule [3] . Inhibition of nitrification can increase the efficiency usage of photosynthesis so it can increase the dry weight of the plant. Proven that N uptake in wheat would increase by 35%, if 25% of its fertilizer N was in the form of  [4] . It is expected that the optimization of nitrification can be developed with a variety of efforts such as the selection of the right litter, so as to achieve the welfare of the corn harvest.
[4] . It is expected that the optimization of nitrification can be developed with a variety of efforts such as the selection of the right litter, so as to achieve the welfare of the corn harvest.
Corn is one of the most important food crops after rice and wheat. Corn is the main source of carbohydrates and as an alternative source of food in some developed countries. Indonesian peoples also use corn as a staple food. Corn’s cobs and leaves are also used as a source of carbohydrates for livestock. Corn’s plants can also be taken its oil, made of flour or cornstarch and used as industrial raw material. Corn cobs have rich pentose. Genetically engineered corn has also been widely cultivated [5] . Because of the importance of this corn cultivation, it must continue to increase in yields and quality, so that it can improve the quality of human resource as a whole.
Nitrification is the oxidation process of 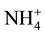 by successive chemoautotroph bacteria produce
by successive chemoautotroph bacteria produce 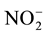 and
and![]() . Bacteria of the genus Nitrosomonas and Nitrospira do nitrification with oxidize
. Bacteria of the genus Nitrosomonas and Nitrospira do nitrification with oxidize ![]() become
become![]() , being bacteria of the genus Nitrobacter do nitrification with oxidize
, being bacteria of the genus Nitrobacter do nitrification with oxidize ![]() become
become ![]() [6] . Nitrification is considered as a detriment because in addition to lowering the efficiency of the utilization of N by plants, it also causes complex environmental problems. Through the nitrification that about 67% of the N fertilizer on various food crops in the world (equivalent to US 15.9 billion dollars/years) will be lost in the form of gases, N2O, NO2, NO and N2, and or lost in the form of nitrate (
[6] . Nitrification is considered as a detriment because in addition to lowering the efficiency of the utilization of N by plants, it also causes complex environmental problems. Through the nitrification that about 67% of the N fertilizer on various food crops in the world (equivalent to US 15.9 billion dollars/years) will be lost in the form of gases, N2O, NO2, NO and N2, and or lost in the form of nitrate (![]() ) to the sub soil layers that can’t be reached by the plant roots [1] . The leaching of
) to the sub soil layers that can’t be reached by the plant roots [1] . The leaching of ![]() will be followed by the leaching of base cations (K+, Ca2+ and Mg2+) resulting in the lower base saturation and increase soil acidity. Factors affecting nitrification are nitrification bacteria populations, the availability of
will be followed by the leaching of base cations (K+, Ca2+ and Mg2+) resulting in the lower base saturation and increase soil acidity. Factors affecting nitrification are nitrification bacteria populations, the availability of![]() , soil pH, and the concentration of base cations, aeration, drainage, moisture, temperature, fertilizer salts as well as the presence of nitrification inhibitor compounds in soils [6] [7] . In developing countries being sought hinder nitrification use slow-release N fertilizer [8] or N fertilizer with nitrification inhibitors such as Thiourea, Sulfathiazole, Dyciandiamide, Etridiazole and N-serve [9] . Application of the synthetic nitrification inhibitor compound shows reduce the loss of soil N effectively, beside the price is expensive (relatively). It also has a detrimental effect on non-target microbes such as N2 fixers (diazotroph) bacteria and mycorhiza fungi [10] . The low potential of soil nitrification in mixed coffee farm is closely related to the diversity of litter quality in surface soil and soil organic content. This indicates that the input of organic matter quality regulate can inhibit nitrification rate, and increase the efficiency of N utilization [11] . The study is designed to optimize the N fertilizer inputs via biological control of nitrification on the corn plant by manipulating the dose and the quality of litter input (lignin and polyphenols content as well as C/N ratio). By balancing the process of N immobilization and nitrification in soil, it is expected to optimize the utilization of N fertilizer inputs and to reduce N loss from the soil, so as to reduce the environmental impact.
, soil pH, and the concentration of base cations, aeration, drainage, moisture, temperature, fertilizer salts as well as the presence of nitrification inhibitor compounds in soils [6] [7] . In developing countries being sought hinder nitrification use slow-release N fertilizer [8] or N fertilizer with nitrification inhibitors such as Thiourea, Sulfathiazole, Dyciandiamide, Etridiazole and N-serve [9] . Application of the synthetic nitrification inhibitor compound shows reduce the loss of soil N effectively, beside the price is expensive (relatively). It also has a detrimental effect on non-target microbes such as N2 fixers (diazotroph) bacteria and mycorhiza fungi [10] . The low potential of soil nitrification in mixed coffee farm is closely related to the diversity of litter quality in surface soil and soil organic content. This indicates that the input of organic matter quality regulate can inhibit nitrification rate, and increase the efficiency of N utilization [11] . The study is designed to optimize the N fertilizer inputs via biological control of nitrification on the corn plant by manipulating the dose and the quality of litter input (lignin and polyphenols content as well as C/N ratio). By balancing the process of N immobilization and nitrification in soil, it is expected to optimize the utilization of N fertilizer inputs and to reduce N loss from the soil, so as to reduce the environmental impact.
2. Method
This research used a Randomized Complete of Block Design (RCBD). We used four types of litter materials selected based on their quality, i.e. lignin, polyphenol, C/N content. Corn seeds use BISI 2 and soils use Alfisol.
Experimental plots were divided into subplots in 3 × 1 m2 dimensions. The basis fertilizers are given simultaneously with the application of litter as a substrate of nitrification. Basis fertilizer dose calculated based on the results of preliminary analysis of soil and nutrient needs of corn crops. Given fertilizer was urea (213.32 kg/Ha), SP 36 (437.81 kg/Ha), and KCl (237.68 kg/Ha). Planting corn spacing was 75 × 25 cm2.
Soil samples for measurement of ![]() and
and ![]() taken at a depth of 0 - 10 cm. Taken soil done intensity as minimum possible as to avoid change the composition of soil. Five grams of soil samples extracted with 20 ml of a KCL 3M solution, shake for 1 minute, add 2 drops of toluene to prevent mineralized and nitrification process, allowed to stand overnight, then filtered with a Whatman-42 filter paper [12] . The concentration of
taken at a depth of 0 - 10 cm. Taken soil done intensity as minimum possible as to avoid change the composition of soil. Five grams of soil samples extracted with 20 ml of a KCL 3M solution, shake for 1 minute, add 2 drops of toluene to prevent mineralized and nitrification process, allowed to stand overnight, then filtered with a Whatman-42 filter paper [12] . The concentration of ![]() and
and ![]() was measured in The Laboratory of Chemistry and Soil Fertility, Faculty of Agriculture, Sebelas Maret University, using spectrophotometer on l 535 mm [13] .
was measured in The Laboratory of Chemistry and Soil Fertility, Faculty of Agriculture, Sebelas Maret University, using spectrophotometer on l 535 mm [13] .
Soil samples for potential nitrification measurements taken by aseptic at a depth of 0 - 20 cm. Nitrification potential was measured from the total of ![]() formed of soil samples after add up with (NH4)2SO4, and incubated at a temperature of 25˚C for 5 hours [14] .
formed of soil samples after add up with (NH4)2SO4, and incubated at a temperature of 25˚C for 5 hours [14] .
The measurement variables of![]() ,
, ![]() , and potential nitrification done once every 2 weeks, which was in week 2nd, 4th, 6th, 8th, and 10th after the litter application. Corn seeds planted on day 2nd after the litter application. The harvest was done in 13 weeks after planting with trim the corn cobs in advance. The data analyzed by analysis of variance test in SPSS for window 17.
, and potential nitrification done once every 2 weeks, which was in week 2nd, 4th, 6th, 8th, and 10th after the litter application. Corn seeds planted on day 2nd after the litter application. The harvest was done in 13 weeks after planting with trim the corn cobs in advance. The data analyzed by analysis of variance test in SPSS for window 17.
3. Results and Discussion
3.1. Doses and Quality of Litter
Litter used in this research content of lignin, polyphenols, and C/N ratio varies (shown in table 1).
Litter decomposition rate is determined by the quality of litter or the content of soluble carbohydrates, amino acids, active polyphenols, lignin, as well as the C/N ratio [15] . High quality litter categorized by ratios C/N less than 25%, lignin <15%, and polyphenols <3% contents, thus they were rapidly decomposes. Based on the lignin content and C/N ratio content, Rice (Oryza sativa) straw and Teak (Tectona grandis) were classified as low quality, where as Angsana (Pterocarpus indicus) litter, and Kirinyu (Eupatorium inulifolium) were classified as high-quality litter. Litter quality differences will result in the release patterns of different ![]() thus it might be able to control the rate of nitrification [16] .
thus it might be able to control the rate of nitrification [16] .
3.2. Dynamic of N
The length of stay ![]() in soil (table 2 and figure 1) can be estimated by calculating the rate value of the mineralized k and 1/k stays of
in soil (table 2 and figure 1) can be estimated by calculating the rate value of the mineralized k and 1/k stays of ![]() (1/k) in soil only about 1.4 to 1.9 weeks except to litter hay reach in 2.1 weeks.
(1/k) in soil only about 1.4 to 1.9 weeks except to litter hay reach in 2.1 weeks.
High-quality litter (Pterocarpus indicus and Eupatorium inulifolium) increase the concentration of ![]() in week 6th after the application.On the other hand, addition of low quality litter (Tectonagrandis and Oryza sativa) caused immobilization of
in week 6th after the application.On the other hand, addition of low quality litter (Tectonagrandis and Oryza sativa) caused immobilization of ![]() from the beginning to the end of experiment (in 10 weeks). Analysis of variance showed a highly significant effect (p < 0.01) in the treatment of type and dose of
from the beginning to the end of experiment (in 10 weeks). Analysis of variance showed a highly significant effect (p < 0.01) in the treatment of type and dose of ![]() soil concentration of the litter on the soil at the various times of incubation. However, there is no real effect of incubation time on soil
soil concentration of the litter on the soil at the various times of incubation. However, there is no real effect of incubation time on soil ![]() concentrations. The addition of low quality litter (Tectona grandis and Oryza sativa) did not increase the concentration of
concentrations. The addition of low quality litter (Tectona grandis and Oryza sativa) did not increase the concentration of ![]() significantly difference compared with the control experiment (no litter and no urea), the average concentration of
significantly difference compared with the control experiment (no litter and no urea), the average concentration of ![]() about 35 mg/Ha (Figure 1).
about 35 mg/Ha (Figure 1).
Concentration of ![]() in second week is higher than in the following weeks. In week 6th and an increased
in second week is higher than in the following weeks. In week 6th and an increased ![]() concentration in low dosage treatment of straw (4 mg/Ha), and the 10th week on the litter of straw in high dosage (16 mg/Ha), begin mineralized. The increased of
concentration in low dosage treatment of straw (4 mg/Ha), and the 10th week on the litter of straw in high dosage (16 mg/Ha), begin mineralized. The increased of ![]() concentration followed by increased of
concentration followed by increased of ![]()
![]()
Table 1. The analysis results of the litter quality.
![]()
Table 2. Rate of ![]() mineralized in soil with various additional litter quality.
mineralized in soil with various additional litter quality.
![]()
Figure 1. ![]() concentration in soil (corrected with atomic weight) after the addition of various qualities and dosage of litter.
concentration in soil (corrected with atomic weight) after the addition of various qualities and dosage of litter.
in soil with the pattern varies between species. High concentration of ![]() in the early incubation (week 2nd) on the whole treatment thought to derive from the basic fertilizer urea hydrolysis. The high concentration of
in the early incubation (week 2nd) on the whole treatment thought to derive from the basic fertilizer urea hydrolysis. The high concentration of ![]() on the second week also thought to derive from the basic fertilizer urea hydrolysis. In week 2nd and 4th, litter treatment Pterocarpus indicus in high quality (N = 2.36%; (L P)/N = 5.8) and in high dosage (4 mg/Ha) increased NH4+ concentration from 42 to 50 mg/Ha, or increased from 90% to 361% of the amount percent in the control experiment (from 21.9 to 10.85 mg/Ha) which only added urea fertilizer (for control experiment). However, in week 6th after incubation,
on the second week also thought to derive from the basic fertilizer urea hydrolysis. In week 2nd and 4th, litter treatment Pterocarpus indicus in high quality (N = 2.36%; (L P)/N = 5.8) and in high dosage (4 mg/Ha) increased NH4+ concentration from 42 to 50 mg/Ha, or increased from 90% to 361% of the amount percent in the control experiment (from 21.9 to 10.85 mg/Ha) which only added urea fertilizer (for control experiment). However, in week 6th after incubation, ![]() concentration is declining for all treatments.
concentration is declining for all treatments.
The concentration of ![]() in soil is precisely the lowest concentration at week 2nd, and increased to peak in week 6th (Figure 2). Decreasing
in soil is precisely the lowest concentration at week 2nd, and increased to peak in week 6th (Figure 2). Decreasing ![]() concentration accompanied by increasing
concentration accompanied by increasing ![]() concentration indicated transformation process of
concentration indicated transformation process of ![]() to
to ![]() in the soil. The results of variance analysis showed that the differences in
in the soil. The results of variance analysis showed that the differences in ![]() concentration were highly significant (p < 0.01) in an incubation time, dosage and type of litter given by. In various types and dosage of litter, an increase in the formation of
concentration were highly significant (p < 0.01) in an incubation time, dosage and type of litter given by. In various types and dosage of litter, an increase in the formation of![]() , real start in week 2nd to week 4th and 6th. Litter of Tectona grandis (low quality) always generates average of low
, real start in week 2nd to week 4th and 6th. Litter of Tectona grandis (low quality) always generates average of low ![]() concentration of soil (145 mg/kg) than any other litter respectively from 219 mg/kg (Oryza sativa), 309 mg/kg (Pterocarpus indicus) and 246 mg/kg (Eupatorium inulifolium). Pterocarpus indicus treatment resulting formation of
concentration of soil (145 mg/kg) than any other litter respectively from 219 mg/kg (Oryza sativa), 309 mg/kg (Pterocarpus indicus) and 246 mg/kg (Eupatorium inulifolium). Pterocarpus indicus treatment resulting formation of ![]() (893 mg/kg).The higher dosage of high quality litter, the higher the amount of
(893 mg/kg).The higher dosage of high quality litter, the higher the amount of ![]() formed.
formed.
Indicated that the process has not been held fast immobilizes ![]() each section of 8 C assimilated during the decomposition of organic matter, soil microbes need about 1 part of N to form the new growth in biomass (or with the ratio C/N = 8/1). It was also stated that the whole of C assimilated only about 1/3 of it used to the cell builder and the rest would be released as CO2. Therefore every 24 grams of C, the soil microbes need about 1gr of N so that the addition of organic matter with high C/N ratio (C/N > 24) into the soil will result in immobilize of
each section of 8 C assimilated during the decomposition of organic matter, soil microbes need about 1 part of N to form the new growth in biomass (or with the ratio C/N = 8/1). It was also stated that the whole of C assimilated only about 1/3 of it used to the cell builder and the rest would be released as CO2. Therefore every 24 grams of C, the soil microbes need about 1gr of N so that the addition of organic matter with high C/N ratio (C/N > 24) into the soil will result in immobilize of ![]() from the soil solution by microbes. The concentration of
from the soil solution by microbes. The concentration of ![]() in soil was determined by the amount of fertilizer addition of
in soil was determined by the amount of fertilizer addition of ![]() or organic materials, absorption roots, microbes, and immobilization or the magnitude of the rate of nitrification in the soil [17] . Synthesis of
or organic materials, absorption roots, microbes, and immobilization or the magnitude of the rate of nitrification in the soil [17] . Synthesis of ![]() will increase in accordance with the availability of
will increase in accordance with the availability of ![]() in the soil. However, the increasing is not necessarily proportional to the availability of
in the soil. However, the increasing is not necessarily proportional to the availability of![]() , and time of occurrence of nitrification also depends on the presence of bacterial nitrification and soil conditions. Nitrification bacteria grow very slowly, so often nitrification took place after the lapse of a few days from the addition of
, and time of occurrence of nitrification also depends on the presence of bacterial nitrification and soil conditions. Nitrification bacteria grow very slowly, so often nitrification took place after the lapse of a few days from the addition of ![]() in soil [18] .
in soil [18] .
3.3. Potential Nitrification of Soil
Potential nitrification were measured by the number of ![]() formed in soil samples after enriched by (NH4)2SO4 as nitrification substrates and incubated for 5 hours (figure 3).
formed in soil samples after enriched by (NH4)2SO4 as nitrification substrates and incubated for 5 hours (figure 3).
Analysis of variance results showed a difference of species/quality and time of incubation effect quite significant (p < 0.01), whereas the dosage litter affect significant (p < 0.05) against potential nitrification of soils. The increasing of potential nitrification in soils is closely connected and significantly (r = 0.802) increased concentrations of ![]() in soil. Dynamics potential nitrification caused by adding with low quality litter is different from high quality litter. Adding low quality litter (Tectona grandis and Oryza sativa), only at very high dosage showed increasing soil nitrification potential as well as 90 % of nitrification potential in initial conditions (22 mg/
in soil. Dynamics potential nitrification caused by adding with low quality litter is different from high quality litter. Adding low quality litter (Tectona grandis and Oryza sativa), only at very high dosage showed increasing soil nitrification potential as well as 90 % of nitrification potential in initial conditions (22 mg/![]() /kg/hour) with low dosage. The key to control nitrification was by controlling the release of
/kg/hour) with low dosage. The key to control nitrification was by controlling the release of ![]() into the soil and by maintaining soil pH of H2O about 4.5 to 5.0. Because that liming needed to be considered, it would improve the process of nitrification in soil.
into the soil and by maintaining soil pH of H2O about 4.5 to 5.0. Because that liming needed to be considered, it would improve the process of nitrification in soil.
![]()
Figure 2. ![]() concentration in soil after the addition of various qualities and dosage of litter.
concentration in soil after the addition of various qualities and dosage of litter.
![]()
Figure 3. Soil nitrification potential after addition of various types of quality and dosage of litter.
On the field condition, N which is not absorbed by the corn plant rooting potentially lost leached to the lower layers. ![]() leaching will bring as well as base cations [6] [19] -[21] .
leaching will bring as well as base cations [6] [19] -[21] .
4. Conclusion
The conclusion of this research is that the management of the quality of the litter input can be applied in the field to hinder the nitrification process of soil N from leaching and improving the efficiency of the utilization of N on corn plants.
Acknowledgements
We thank Higher Education Competitive Research Project Ministry of Education and Culture Republic of Indonesia for Grand Featured Research Mail Agreement No. 2342/UN 27.16/PN/2012 State Budget Funds and No. 159a/UN 27.11/PN/2013 and especially thank Prof. Dr. Rafik Karsidi, MSc as a Rector of Sebelas Maret University Surakarta Indonesia; Prof. Dr. Ir. Darsono, M.Si as a Chairman of the Institute for Research and Community Service; Prof. Dr. Ir. Bambang Pujiasmanto, M.Si as a Dean of Agriculture Faculty of Sebelas Maret University Surakarta Indonesia and Dr. Adi Prayitno, DDS, PhD for all kindness.