1. Introduction
Separation of suspended solids from water is an important stage of water cleaning technology. When sorption process is used to remove pollutants from water, the highest sorption capacity of the powder sorbent is reached for the smallest particles with diameters in nanosized range. However, the difficulty in separating the solid phase from the water suspension using conventional mechanical filtration and sedimentation methods is increased correspondingly. The conventional adding of coagulants brings about secondary pollution.
If the nanoparticles have magnetic moments, their separation from aqueous solution can be enhanced by gradient magnetic fields. The most prospective magnetic nanosorbents for this purpose are iron and magnetic iron oxides―magnetite, γ-Fe3O4 and maghemite, γ-Fe2O3 [1] as they are environmentally friendly, not expensive in bulk, and so currently many methods of synthesis of iron oxide nanoparticles have been developed [2] - [4] . Magnetite Fe3O4 nanoparticles (NP) adsorb efficiently heavy metals, actinides, organic and mineral impurities. Surface modified magnetic nanoparticles can adsorb viruses and other biological objects [1] [5] [6] . While many contributions are devoted to the sorption process, the subsequent separation of the nanosorbent from water is poorly investigated [1] [7] so far. In our previous papers [8] [9] , the sedimentation of the nanoparticles (10 - 100 nm) in water in a gradient magnetic field produced by two sets of permanent magnets was studied. There are also ways to increase the magnetic field gradient by a steel grid with sharp edges.
The sedimentation of magnetite particles in water is a complex process due to aggregation formation. According to the DVLO model, the stability of a colloid system under an external magnetic field is defined by the minimum of the sum of main interactions: electrostatic interactions energy, van der Waals energy and energy of magnetic interactions [10] . Under the action of these forces, the small particles can form the large aggregates in aqueous media. If the particles carry magnetic moments, as in the case of magnetite, magnetic attraction forces are very strong and exceed other interactions. Electrostatic forces depend on the charge of the double layer on the particle surface and can be varied by the sign and by the value when pH of aqueous media is changed. The DLS studies [8] revealed that in water in the gravity field, 80 nm-nanoparticles form aggregates with the micron sizes which slightly change on acidity variation in the range 5 < pH < 9. At the same time, it has been shown that the sedimentation speed of the magnetite nanoparticles doesn’t change significantly. Correspondingly, the effect of pH change on the sedimentation dynamics of magnetite nanoparticles (80 nm) is pronounced, but still much less than the effect of magnetic field.
Magnetic attraction forces are defined by the magnetic moments of interacting particles which depend on the material and on whether the particles are in a ferromagnetic or in a superparamagnetic state. From magnetization data and Mössbauer spectra [11] , for magnetite Fe3O4, the border line particle diameter between a ferromagnetic and a superparamagnetic states is dsp ≈ 5 nm. The particles with d < dsp slightly aggregate in water, and the water suspension remains stable for a long time (months, years). Also, they are comparatively weakly magnetized in external magnetic field (B < 0.5T) at 300 K and to let them settle down rather strong magnetic fields (of the order several T) and high gradients are required [12] . Therefore, the optimal size of the particles in the powder sorbent should be a little larger than the superparamagnetism criteria for the material.
In this work, the sedimentation dynamics of magnetite nanopowders with the particle sizes of 10 - 20 nm in aqueous media in the presence of a gradient magnetic field B ≤ 0.3 T, dB/dz ≤ 0.13 T/cm was studied by optical and Nuclear Magnetic Resonance (NMR) relaxometry methods. The NMR relaxometry method allows to register fast the residual magnetite powder concentration in water after the sedimentation process. The height of the water layer (h) was chosen 20 mm, which provides stronger magnetic field intensity and magnetic field gradient in vertical direction as compared with the heigh 30 mm in our previous study. The starting solid phase concentration (c0) was varied from 0.1 g/l to 1 g/l. In contrast to still water, the preliminary experiments were done for flowing water conditions. This study will be helpful for optimization of nanosorbent sedimentation conditions for practical application.
2. Experimental Section
The powder samples of Fe3O4 were prepared by gas condensation method [13] and were examined by X-ray- method, the transmission electron microscopy (TEM). From the magnetization measurements and the Mossbauer spectra the magnetic state of the powders was detected. The main phase (95%) has a distorted cubic structure of the magnetite (Fe3O4), the secondary phase is α-Fe presented in the kernel of some particles. From the TEM images the size-distribution histograms N(d) were obtained (e.g. Figure 1(b)). The specific surface, determined by the Argon gas desorption BET method, corresponds to the detected sizes of their main fractions 10 - 20 nm. Magnetic measurements give the magnetization value at room temperature of 60 - 70 emu/g, which is 20% - 30% lower than that in a bulk magnetite (92 emu/g), which is typical for nanopowder state of the magnetite.
![]()
![]() (a) (b)
(a) (b)
Figure 1. Characterization of the magnetite nanoparticles. (a) TEM image and SAED-pattern; (b) The size-distribution histogram N(d).
The size of the magnetite particles in water (dh) was determined from Dynamic Light Scattering (DLS) experiments by using NanoZS apparatus (Malvern, UK). Laser beam λ = 633 nm was produced by He-Ne laser, operating in back-scattering mode at an angle of 173˚.
The magnetic field source (magnetic system) was made from a set of permanent magnet bars Sm2Co17 (10 *40 *40 mm) with a remanence induction Br = 1 T. The bars were magnetized in plane in opposite directions. Between the bars, strips of the same thickness made from magnetically soft steel were inserted. The sedimentation was studied in water layers: h = 20 mm, where magnetic field B ≤ 300 mT, with maximal gradient 0.13 T/cm was acting in vertical direction on magnetite particles. In some cases, a grid from magnetically soft steel was placed beneath the cuvette containing the suspension to enhance the magnetic field gradient. The grid was 0.1 mm thick with the holes 2 *2 mm with the sharp edges, which resulted in a gradient increase near the grid up to 5 T/cm (estimated).
Before the sedimentation dynamics study, suspensions with different starting magnetite powder concentration of 0.1 - 1 g/l in distilled water were prepared, with subsequent excitation by ultrasonic probe for 10 seconds. As a rule, for each sedimentation measurement a freshly made suspension was used.
The sedimentation dynamics of the magnetite nanopowder (d ≈ 10 - 20 nm) was registered by optical method by monitoring the light transmission coefficient k. In optical set up, a monochrome light beam with 950 nm wavelength in horizontal direction was split into two beams, one going through pure water and another going through a fluid optical cell containing the magnetite suspension. Then the beams were detected by a photodiode and the ratio between the signals was amplified and PC-monitored versus time. Before each sedimentation experiment, calibration of the optic cell using pure transparent water was performed. Under the optical cell with the magnetite suspension the magnetic systems could be placed.
In order to determine the residual concentration of magnetite in water after sedimentation, the method of Nuclear Magnetic Resonance (NMR) relaxometry was explored. This method is based on the registration of the relaxation time T2 of the NMR echo signal from the hydrogen nuclei in water (H+). The impurities bearing magnetic moment, dispersed in water, influence the local magnetic field gradient near the hydrogen nuclei, which is reflected in a change of T2. For the water samples taken from the upper layer of the cuvette, T2 was measured using the NMR relaxometer with the resonance frequency 2.06 МHz. The values of Fe3O4 content in water were determined using a calibration dependence 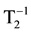 versus c(Fe3O4). From the c(Fe3O4) values the values of c(Fe) were calculated. Figure 2 shows the calibration curve obtained for the given Fe3O4 nanopowder concentrations. For small magnetite concentrations, (c << 0.001 mg/l) the values of relaxivity can be extrapolated to 2.2 s, which corresponds to pure water. The values c(Fe) determined by NMR relaxometry method are in agree- ment with the data obtained by ICP analysis and by magnetic measurements of the water suspension samples.
versus c(Fe3O4). From the c(Fe3O4) values the values of c(Fe) were calculated. Figure 2 shows the calibration curve obtained for the given Fe3O4 nanopowder concentrations. For small magnetite concentrations, (c << 0.001 mg/l) the values of relaxivity can be extrapolated to 2.2 s, which corresponds to pure water. The values c(Fe) determined by NMR relaxometry method are in agree- ment with the data obtained by ICP analysis and by magnetic measurements of the water suspension samples.
Statistical errors are increasing for c(Fe) < 0.05 mg/l, due to increasing influence of water composition inhomogeneities (in particular, absorbed gases) and due to increased uncertainties in the NMR signal mathematical processing. For higher magnetite concentrations, c > 360 mg/l, the T2 values are rather low, close to 2 - 3 ms,
![]()
Figure 2. Dependence of the relaxivity 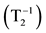 of the hydrogen nuclei on the iron concentration in the magnetite nanopowder water suspension.
of the hydrogen nuclei on the iron concentration in the magnetite nanopowder water suspension.
which gives big errors ±2 ms. For the comparatively high magnetite concentrations, the nanoparticles are magnetized in the own magnetic field of the device and aggregate and sediment fast, thus being excluded from the region from which the NMR signal is registered. The observed slight nonlinearity of the 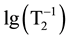 on lg(c(Fe)) dependence might be accounted by a decrease of T2 values because of aggregates formation, as it has been studied earlier [14] .
on lg(c(Fe)) dependence might be accounted by a decrease of T2 values because of aggregates formation, as it has been studied earlier [14] .
3. Results and Discussion
The sedimentation rate of nanoparticles in water results from the sum of forces of different nature and strength: gravity, buoyancy, viscous resistance, thermal motion. By settling down in water in the gradient magnetic fields two additional effects are important: 1) particle magnetization enhances their attraction, hence, aggregates with large magnetic moments can be formed, and 2) the magnetic force directed to the region of the gradient increase is added to the gravity force. As the aggregation process is crucial for the sedimentation dynamics, one can expect the influence of interparticle distances on the aggregates formation, in particular, for the different solid face concentrations.
Figure 3(a) and Figure 3(b) show the comparison of the sedimentation curves and the water transparency for the magnetite nanopowder suspension in the gravitation field only, and in the presence of a gradient magnetic field.
While in the gravitation field only, it will take more than 24 hours for beginning of the sedimentation process for suspension of such particles [8] , under the gradient magnetic field about 80% of the particles sediment for the first 20 minutes. This reflects a significant effect of a gradient magnetic field on the sedimentation dynamics.
As the optical registration of transparency/turbidity for t > 50 min can not supply a good precision, the residual concentration of the magnetite nanoparticles in the water was measured by NMR relaxometry as it is shown in the Figure 4 where the comparison of water clarification dynamics for different starting magnetite concentration c0 is presented.
There is a pronounced difference in sedimentation dynamics for higher and lower starting concentration suspensions. For the first hour of settling of suspension with c0 = 1 g/l, the iron concentration decreases by 20%, while for the suspension with c0 = 0.1 g/l the iron concentration decreases by 50%. Figure 4(a) shows that the sedimentation rate depends on c0, the difference is the strongest at the beginning, for t < 10 min. The dependence of the initial sedimentation rate for different time periods is plotted versus c0 in the Figure 4(b). One can see that by going from c0 = 1 g/l to lower concentration the sedimentation rate firstly decreases, then, for c0 < 0.5 g/l it increases, which is rather surprising.
Such behavior does not agree with the data [10] , for sedimentation in the gravitation field only where more
![]()
![]() (a) (b)
(a) (b)
Figure 3. (а) Sedimentation dynamics for Fe3O4 nanopowder in the gravitation field only (1), under gradient magnetic field Bmax = 300 mT, dB/dz ≤ 0.13 T/cm (2), under gradient magnetic field enhanced by steel grid Bmax = 30 mT, dB/dz = 5 T/cm (B + grid) (3); (b) Photos illustrating the transparency of the water in the absence and in the presence of the gradient magnetic fields (B = 0, B, B + grid) upper panel t = 5 min, low panel t = 180 min.
![]()
![]() (a) (b)
(a) (b)
Figure 4. (a) The time dependence of the residual concentration of iron in water after the magnetite nanoparticles sedimentation in the gradient magnetic field for different starting powder concentration c0; (b) Relative magnetite concentration change for different initial time periods.
rapid sedimentation was observed for more concentrated suspensions than for more diluted ones. It seems that in magnetic field at higher magnetite powder concentrations, colloid formation occurs, with a structure which sediments slower in comparison with sedimentation of big aggregates formed in diluted suspension. The kinetic behavior of a dispersion with magnetic particles of micron sizes was studied theoretically and experimentally [15] [16] . It was shown that in magnetic colloids the particles are assembling in dimers, trimers and chains oriented along the external magnetic field direction [17] . At higher concentrations, more complex structure can be built, in particular, with magnetic flux closing, that is a sort of a fractal structure as a network, so called gel structure [18] [19] .
The nonlinear dependence Δc/c0 on starting powder concentration might be the evidence that for higher powder concentrations, that is, c > 0.5 g/l, a gel structure is formed, therefore its settling is similar to the settling in the absence of magnetic field, that is in accordance with the results of other researchers [10] .
The effect of magnetic field on sedimentation dynamics for different regimes is illustrated in the Figure 5. The starting sedimentation rate significantly increases by placing the grid, which enhances the magnetic field
![]()
Figure 5. Time dependence of the residual iron concentration in water by sedimentation under different regimes. (the starting nanopowder concentration c0 = 1 g/l).
gradient. This is consistent with the force acting on the magnetic particle in a gradient magnetic field:
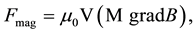
where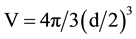 ―is the volume of the particle, M―magnetization in a given magnetic field B, μ0― permeability in vacuum.
―is the volume of the particle, M―magnetization in a given magnetic field B, μ0― permeability in vacuum.
Within the first 10 minutes, the iron concentration drops down to 36 mg/l, that is, decreases by 20 times. After 180 minutes the concentration reaches a value of 0.4 mg/l, coming close to the hygienic and environment standard for iron concentration in drinking and fishery waters 0.3 mg/l [20] .
To extend the time of particle interaction with magnetic field gradient, it is reasonable to make water flow in several circles. Also, it might be expected that in flowing water the sedimentation speed will increase due to larger aggregate formation in the course of intensified particle collisions. The suspension circulated in a closed system, passing through the reactor, being clarified in the presence of a gradient magnetic field, and returned again to the glass container. Time of the cycle depended on the water flow speed.
In the flowing water conditions the highest sedimentation rate is observed in comparison with still water. For the water flow speed of 25 mm/min, one cycle lasts for about ten minutes. Thus, the iron concentration of 0.3 mg/l is reached after 80 minutes and it is reduced more for longer times. The more detailed studies of the nanoparticle sedimentation in flowing water are in progress.
4. Conclusions
Sedimentation dynamics of magnetite nanoparticles (10 - 20 nm) was studied in the gradient magnetic field for different starting nanopowder concentrations c0 = 0.1 - 1 g/l. Gradient magnetic field, produced by a system of strip permanent magnets (Bz ≤ 0.3 T, dB/dz ≤ 0.13 T/cm) and, in some cases, enhanced by a steel grid with sharp edges, speeds up the sedimentation process. The initial sedimentation rate of the nanoparticles in water and under magnetic fields is higher for less concentrated suspensions (c0 = 0.1 g/l) than for more concentrated ones (c0 = 1 g/l), which might be connected with the formation of gel structures due to strong magnetic attraction between ferromagnetic nanoparticles.
In the gravitational field only, the magnetite nanoparticle (10 - 20 nm) suspension stays stable for several hours. Under gradient magnetic fields of strip magnets, the magnetite particle concentration in the water layer of 20 mm is reduced by 80% within the first minute. The sedimentation of nanopowder in a gradient magnetic field enhanced by a steel grid for 180 minutes resulted in the reduction of the iron concentration in water down to 0.4 mg/l, which is close to hygienic and environmental standards for drinking water and fishery. In the flowing water regime (25 mm/min) the residual iron concentration in water 0.3 mg/l is reached after 80 minutes. The more detailed recommendation for working device will require substantial studies to find optimization between magnetic field strength and gradient, water layer height, water flowing speed, nanoparticle starting concentration.
Acknowledgements
The work was supported from the grant of the Russian Academy of Sciences. The authors thank V. Gaviko and L. Stashkova for XRD analysis.