1. Introduction
Conversion of agricultural wastes to ethanol presents an important opportunity to reduce energy costs. Compared to other forms of biofuels (biogas, biodiesel) delignification of lignocellulosic biomass becomes important because they are plentiful and inexpensive materials [1] . Technologies for the conversion of cellulose to ethanol are already abundant. It is the lignin component of the ligninocellulosic materials that hinders enzymatic access to cellulose [2] .
Lignocellulose is a structure composed of lignin, cellulose and hemicellulose and represents the most abundant renewable organic resource from the soil. When bound to cellulose and hemicellulose, lignin forms a barrier that reduces the degradation of lignocellulosic materials and complicates the industrial use of plant polysaccharides in paper and biofuel production, among other processes [3] [4] .
The biological process involved in the conversion of lignocellulosic residues to high value products requires the following: 1) a delignification pretreatment (i.e., mechanical, chemical or biological) to release cellulose and hemicellulose from their complex, 2) the depolymerization or hydrolysis of carbohydrate polymers to produce metabolizable molecules (i.e., free sugars: hexoses and pentoses), 3) the use of these molecules to support microbial growth or obtain chemical products, and 4) the separation and purification of the obtained products. The delignification process of lignocellulosic feedstocks is considered the most difficult step and is the limiting step in the conversion [1] [3] .
The biological delignification process is primarily performed by basidiomycete fungi: a large group with over 30,000 species that includes brown and white rot fungi. The later are organisms capable of efficiently degrading and mineralizing lignin to CO2 and water, which is termed “enzymatic combustion” [5] . Species of the genus Trametes are among the most efficient degraders and their ligninocellulolytic enzyme system is comprised of laccase and MnP, as well as a series of cellulases and cellobiose dehydrogenases [6] .
Mn-dependent peroxidase (or manganese peroxidases―MnP) (EC 1.11.1.13) are extracellular glycoproteins that contain a heme prosthetic group and show molecular weight ranging from 45 to 47 KDa. Currently, MnP is considered the key enzyme in lignin degradation. The secretion of this enzyme depends on environmental factors and is strongly regulated by nutrients availability [7] [8] .
Ligninolytic systems have been extensively studied in recent years, and researchers agree that the type and composition of the substrate appears to determine the amount of enzymes produced by basidiomycetes. Additionally, the temperature, pH, carbon source and nitrogen affect the growth of the fungus and ligninolytic activity [9] - [12] .
According to Arantes et al. [13] , the response surface methodology is a powerful and efficient mathematical approach widely applied in the optimization of fermentation processes. Therefore, many authors applied this methodology to evaluate enzyme production by fungi [14] - [17] .
This study was performed to optimize the production of MnP by the basidiomycete fungus Trametes villosa. The subsequent application of the enzyme extract was to determine its potential for the delignification of sugarcane bagasse, coconut shell and sisal fibers, which are abundant agricultural wastes in Northeastern Brazil.
2. Materials and Methods
2.1. Microorganism
Ten unidentified fungi isolates were tested for their ability to decolorize Remazol Brillinat Blue R (RBBR) dye in Petri dishes containing agar and the dye, according to methodology described by Palimeri et al. [18] . The T. villosa isolate showed the larger decolorization halo and was thus selected for identification and further studies (data not shown).
The fungal strain used was Trametes villosa (Sw.) Kreisel CCMB 651 deposited in the Culture Collection of Microorganisms of Bahia―CCMB, Feira de Santana, BA, Brazil (http://www.uefs.br/ccmb) [19] . This strain was isolated from tissue of the internal cortex region of the pileus of field-collected basidiomata (HUEFS 108280) of the Brazilian semi-arid region (Serra das Candeias, Quijingue, Bahia, Brazil, 10˚55'16''S and 39˚4'30''W), deposited in the Herbarium of the State University of Feira de Santana (http://herbario.uefs.br). The morphological identification of this voucher specimen was reviewed [20] and its rDNA ITS region was sequenced to be used as one of DNA barcodes of Trametes villosa in BrBOL (Brazilian Barcode of Life Project, www.brbol.org). Trametes villosa is easily distinguished from other species of Trametes due to the flexibility of their basidiomata and the large pores of the hymenophore. More than 40 synonyms are known for T. villosa and a list of all of them can be found in Mycobank (http://www.mycobank.org).
The medium used for the activation and growth of the isolate consisted of wheat grain containing calcium carbonate and was incubated at 28˚C in a biological oxygen demand (BOD) incubator for seven days. The plates were stored at 4˚C and the subcultures were performed every three months in duplicate.
2.2. Optimization of Culture Conditions for MnP Production
The experiments were based on a central composite rotatable design using Statistica (6.0) software, in which three independent variables (i.e., temperature, moisture content and pH) were evaluated at five levels according to Table 1. The response variable analyzed was the specific activity of MnP. In previous studies, sugarcane bagasse provided the best results for fungus growth and enzyme production (data not shown) compared to the other wastes tested (sisal fiber and coconut shell). Thus, it was selected for further studies.
The fixed conditions for all assays were as follows: 250 mL conical flasks containing 20 g of sugarcane bagasse (chopped, yielding little cubes of 0.5 cm side), an inoculum of five grains of wheat showing mycelium with seven days of growth and a cultivation time of 14 days. The initial moisture of the bagasse was adjusted for 50% by drying in a ventilated oven and the final moisture used in the assays (Table 1) was reached by the addition of buffer in the corresponding pH value (Table 1).
2.3. MnP Extraction and Assay
After the culture period, an aqueous extraction of the enzymes was performed from the fermented biomass, i.e., 50 mL of cold distilled water was added to the medium and the mixture remained in an ice bath for one hour with occasional stirring. The content was then filtered through gauze and centrifuged at 4˚C and 5000 × g for 10 minutes. The supernatants were separated into aliquots, frozen and stored for the determination of enzyme activity.
The MnP activity was determined by the oxidation of phenol red in the presence of hydrogen peroxide according to the modified methodology of Kuwahara et al. [21] . The reaction medium was composed of 500 μL of the crude enzyme extract, 50 μL of manganese sulfate (2.0 mM), 200 μL of bovine albumin (0.5% w/v), 50 μL of hydrogen peroxide (2 mM) in sodium succinate buffer (0.2 M, pH 4.5), 100 μL of sodium lactate (0.25 M) and 100 μL of phenol red (0.01% w/v). The reaction was monitored by reading the absorbance at 610 nm in a UV-Vis spectrophotometer (Varian Cary 50), which was recorded every 10 seconds for five minutes and then stopped by the addition of 40 μL of a sodium hydroxide solution (2.0 M). After termination, the absorbance was monitored for another minute. The blank consisted of the boiled crude extract at 100˚C for 10 minutes prior to centrifugation. The analyses were performed in triplicate.
The activity of MnP (U/L) was calculated using Equation (1), as described by Menezes, Silva and Durrant [22]
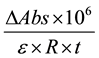 (1)
(1)
where DAbs is the difference between the absorbance of the boiled extract and non-boiled extract (i.e., the blank and test) at 0 and 5 minutes; e is the extinction coefficient of oxidized phenol red at 610 nm = 4460 L, m−1・cm−1 ;
![]()
Table 1. Independent variables and their levels for the optimization of MnP production by T. villosa (Sw.) Kreisel CCMB 651 in solid-state fermentation.
*Value of α for experiments with three independent variables.
R is the aliquot of crude enzyme extract (mL); and t is the reaction time (min).
The specific activity was calculated from the quotient of enzyme activity by the protein concentration determined by the Lowry method [23] .
2.4. Enzyme Secretion over the Incubation Period
According to the results obtained after the statistical evaluation of the optimization experiments, another experiment was set up to verify the influence of incubation time, applying the optimized conditions, on the secretion of MnP. The enzyme activity was measured at 5, 10, 15, 20, 25 and 30 days of fungus growth in conical flasks (250 mL) containing 20 g of sugarcane bagasse, at 80% moisture content and pH 9.4, incubated at 20˚C in a BOD. The cultivation was conducted in duplicate and the enzyme activity was evaluated in triplicate. The crude protein content of the enzyme extract was determined by the Lowry method [23] to calculate the specific activity.
2.5. Delignification of Plant Residues
After defining the optimal culture conditions that favored the secretion of MnP, the crude enzymatic extract was produced and applied for the delignification of sugarcane bagasse, coconut shell and sisal fiber. The reaction medium consisted of 2 g of plant residue, 5 mL of enzyme extract (specific activity: 0.236 U/mg) and 15 mL of sodium succinate buffer (0.2 M, pH 4.5) containing manganese sulfate (2.0 mM), bovine albumin (0.5%), hydrogen peroxide (2 mM) and sodium lactate (0.25 M). The medium was incubated in 250 mL conical flasks at 30˚C and 150 rpm. The reaction times were 4, 12 and 72 hours, and the treated residues were washed with distilled water and frozen for later determination of lignin content. A blank without the enzyme was also perfomed and provided the initial lignin content. All tests were performed in triplicate, and the percentage of lignin removed was determined by Equation (2):
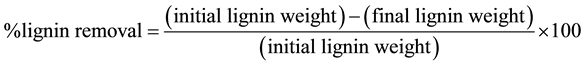 (2)
(2)
2.6. Lignin Content
The lignin content of the samples was determined by the Van Soest method [24] . First, the acid detergent fiber was determined by the digestion of 1 g of the dried and ground sample with 100 mL of the acid detergent solution (cetyltrimethylammonium-bromide 0.2% in sulfuric acid 1 N). This solution was then transferred quantitatively to the coarse porosity filter (100 - 160 microns), and it was washed with hot water and acetone under vacuum filtration. Subsequently, the filter crucibles were dried in an oven at 105˚C for 8 hours.
The crucibles containing the filtered residue from the acid detergent fiber were supplemented with 72% sulfuric acid and subjected to vacuum filtration. The residues were dried in an oven at 100˚C for 8 hours and incinerated in the oven at 550˚C for 3 hours. The lignin content was calculated by the weight loss after incineration.
2.7. Statistical Analysis
The software Statistica (6.0) was applied to construct the table of analysis of variance (ANOVA), response surfaces, contour plots and the predictive model.
3. Results and Discussion
3.1. Optimization of Culture Conditions for MnP Production
According to Elisashvili and Kachlishvili [7] and Cupul et al. [25] the main issue delaying the implementation of ligninolytic enzymes in various biotechnological applications at industrial scale is the low yield of ligninolytic enzymes in most white-rot fungi, because their constitutive extracelullar enzymes are only produced in small amounts. Therefore all the efforts devoted to optimize the production of these enzymes could be regarded as valid. Enzymatic activity in the genus Trametes has been widely studied, however T. villosa has not. Likewise, laccases have been investigated far more than MnP.
In this work, specific activity was evaluated as the response to the optimization of MnP using the software Statistica (6.0) (Table 2). Regression analysis was performed to determine the relationship between the factors and the response investigated. The significant factors affecting the MnP production were the linear effects of temperature and pH ( and
and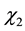 ) as well as the quadratic effects of moisture and pH (
) as well as the quadratic effects of moisture and pH (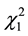 and
and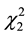 ) (p < 0.5). Considering only the statistically significant terms from the ANOVA, the calculated F value (8.54) for the regression was significant at the 5% probability (F4;13;0.01 = 5.21) and the coefficient R2 (0.7245) indicated that there was a good correlation between the activities determined experimentally and the values predicted by the model (Equation (3)), since 72.45% of the variability in the experimental data could be explained by the estimated model. The response surfaces obtained from the quadratic model could be used to describe the results. The statistically insignificant parameters were removed from the model and added to the residue.
) (p < 0.5). Considering only the statistically significant terms from the ANOVA, the calculated F value (8.54) for the regression was significant at the 5% probability (F4;13;0.01 = 5.21) and the coefficient R2 (0.7245) indicated that there was a good correlation between the activities determined experimentally and the values predicted by the model (Equation (3)), since 72.45% of the variability in the experimental data could be explained by the estimated model. The response surfaces obtained from the quadratic model could be used to describe the results. The statistically insignificant parameters were removed from the model and added to the residue.
Trials 9 and 4 showed greater crude (117.327 U/L) and specific activities (0.167 U/mg), respectively. Trial 9 presented a greater crude activity but a lower specific activity which can be explained by the high protein content of the extract, that was presumably caused by the medium pH (equal to 7) that most likely favored the production of other proteins.
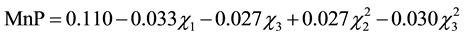 (3)
(3)
Figure 1 and Figure 2 show the three-dimensional relationships between the dependent variables and the specific activity of MnP.
The response surface plot in Figure 1 shows that the decrease in temperature within the range tested had a positive effect on the MnP production: the specific activity was maximized when the temperature tended to a minimum (20˚C). It shows that lower temperatures may produce even better results; however, the strain tested was not able to grow when submited to temperatures below 20˚C.
![]()
Table 2. T. villosa (Sw.) Kreisel CCMB 651 MnP activity (crude and specific) in sugarcane bagasse solid-state fermentation.
*An average absorbance of the three repetitions was used in the calculations.
![]()
Figure 1. Response surface for the influence of moisture content (%) and temperature (˚C) on MnP specific activity.
![]()
Figure 2. Response surface for the influence of pH and temperature (˚C) on MnP specific activity.
Although media composition and growth conditions strongly affect the prodution of ligninolytic enzymes [26] , very few studies linking growth temperature of Trametes sp. and MnP activity are available. Knezevic et al. [6] , however, obtained good results cultivating two species of Trametes at 22˚C. Fernández-Fueyo et al. [26] , when studying the activity of different peroxidases secreted by Pleurotus ostreatus found out that extreme temperature (10˚C and 37˚C) values caused reduction in peroxidase secretion, especially MnP. For P. ostreatus the best growing conditions were at 25˚C and pH = 5.5. Mester and Field (1997) optimized the secretion of MnP by Bjerkandera sp. at 30˚C and pH = 5.2. However, secretion of ligninolytic enzymes by white-rot fungi is belived to be species and strain dependent [25] .
The highest levels of specific MnP activity were achieved for the pH range between 7.0 (0) and 11 (+α) with decreased temperature levels (Figure 2). An increase in the medium pH from 7.0 to approximately 9.0 caused an increase of roughly 50% in the specific activity of MnP. Secretion of oxidative enzymes by fungi can be related to the exposure of the fungal culture to abiotic stress such as elevated temperature [27] , heavy metals [28] , and oxidative stress [29] . Co-cultivation with other microorganisms was also found to cause stress resulting in increased secretion of ligninolytic activity [25] . The cultivation of the T. vilosa isolate on pH 9.0 media may have been a cause of stress responsible for enhancing MnP activity. Wang et al. [30] enhanced laccase production by T. versicolor in acid pH (4.0) as well as Carabajal et al. [31] growing the same species on tomato juice medium. On the other hand, in their work on P. ostreatus, Fernández-Fueyo et al. [26] report that extreme pH values (3.0 and 8.0) resulted in reduced enzyme activity.
Considering the analysis of response surfaces, the following conditions were established for the experiments that followed: pH 9.4, temperature 20˚C and moisture content of 80%.
The maximum MnP activity determined was of the same magnitude as that reported for many basidiomycetes. The MnP was the dominant enzyme produced by Irpex lacteus, with an average activity of 270 U/L in a study conducted by Baborová et al. [32] . The production of MnP by Trametes villosa is rarely described in literature and the concentrations determined in this study were much greater compared to the three strains studied by Machado et al. [33] , who encountered a maximum MnP activity between 1.2 and 6.64 U/L under submerged cultivation in supplemented medium containing sugarcane bagasse. In the submerged culture of Phanerochaete chrysosporium, the maximum activity of purified MnP was achieved in 64 h (0.5 U/mL or 500 U/L), after which it began to decrease [34] . In the liquid culture of Stropharia coronilla, an activity of 0.77 U/mL (700 U/L) was determined after 45 days of cultivation [35] . Lopez et al. [36] determined a maximum activity of 0.22 U/mL (220 U/L) in Coniochaeta ligniaria after 10 days of cultivation under the solid fermentation of pepper plant residues. In Trametes villosa, under liquid fermentation with glucose supplementation, the MnP activity peaked at 6 U/L after 27 days [37] .
Elisashvili et al. [38] showed that solid-state fermentation (SSF) of plant substrates is favorable for MnP secretion, whereas submerged fermentation (SF) decreases or inhibits this enzyme production by L. edodes and several Pleurotus strains. This finding is in accordance with other reports indicating that MnP productivity in SSF is far higher than that in SF of plant raw materials [39] [40] . Vassilev et al. [41] evaluated the influence of initial temperature, pH and humidity under the production of MnP by Phanerochaete chrysosporium on dry olive and sugar beet wastes SSF. The results showed 37˚C, 60% humidity and pH 5.0 as optimum conditions for MnP secretion. The highest activity found was around 1100 U/L after a 21-day process. Papinutti and Forchiassin [42] studied the MnP production by Fomes sclerodermeus growing in SSF performed by using soy and wheat bran. The maximal MnP activity occurred after 15 days post-inoculation. The MnP activity is largely variable according to the studied strain. Besides, the nature of lignocellulosic material and the method of fungi cultivation, including important factors like temperature, humidity and pH are determinant for the expression of ligninolytic potential of fungi.
3.2. Enzyme Secretion over the Incubation Period
The specific MnP activity increased until the 15th day, after which it decreased until approaching zero on 30th day. The analysis of variance with the Tukey post-test for the comparison of means indicated significant differences at the 15th day compared to all others. Therefore, 15 days was considered the best time for the secretion of MnP using the optimized conditions for Trametes villosa (Table 3).
Cupul et al. [25] achieved maximum MnP activity in cultures of T. maxima at the 10th day of growth; Irbe et al. [12] detected higher MnP activity after 14 days cultivation of T. versicolor; Knezevic et al. [6] found maximum MnP activity after 14 days of growth of T. multicolor, Elisashvili and Kachlishvili [6] optimized MnP secretion by T. pubescens after 11 days of solid-state fermentation. This shows that T. villosa exhibits MnP activity peak after similar cultivation time to other species of the genus.
3.3. Delignification of Plant Residues
The percentage of lignin removal for plant residue after the reaction with the crude enzyme extract containing MnP is presented in Table 4. Although only the MnP activity was optimized, other enzymes secreted by the isolate may have had part in the delignification.
![]()
Table 3. Specific activity of manganese peroxidase produced by T. villosa (Sw.) Kreisel CCMB 651 in sugarcane bagasse solid-state fermentation along the incubation period.
Culture conditions: 20 g of sugarcane bagasse at 80% humidity, pH 9.38 and 20˚C. The activity values are the average of three repetitions. The mean values with the same letters do not differ among themselves according to the Tukey test.
![]()
Table 4. Lignin removal of sugarcane bagasse, coconut shell and sisal fiber by crude enzymatic extract obtained from T. villosa (Sw.) Kreisel CCMB 651 in sugarcane bagasse solid-state fermentation.
*The mean values with the same letters do not differ among themselves according to the Tukey test.
The crude enzyme extract caused a reduction in lignin content for all residues tested: 35.05 ± 1.45 (%) for the sugar cane bagasse; 63.11 ± 0.06 (%) for the sisal fiber and 39.61 ± 0.39 (%) for the coconut shell, under the reaction conditions, after 4 hours of fermentation. No improvement was detected for longer reaction times.
There are different delignification processes applied as pretreatments for bioethanol production [1] . Thermal and/or chemical pretreatments have been regarded as the current leading pretreatment technologies; however, they rely on expensive equipment and usually require elimination of compounds toxic to the fermenting microorganisms that will benecessary to produce ethanol. Biological pretreatments, applying lignin degrading microorganisms such as white-rot fungi, can be very efficient and show lower energy requirements and reduced inhibitors to ethanol fermentation than thermo-chemical pretreatmens. However, substantial cellulose and hemicellulose loss and long time are main difficulties of this form of treatment [43] . Despite these disadvantages, several authors achieved high levels of delignification when working on fungal solid state fermentation of ligninocellulosic materials. Arora et al. [44] reached 30.6% lignin degradation in wheat straw; Wang et al. [30] managed to achive 34.7% of lignin degradation in rice straw with little (2,1%) holocellulose degradation. Knezevic et al. [6] removed 5.9% of the lignin in oak sawdust and Deswal et al. [45] degraded lignin up to 7.91% in sugarcane bagasse. Although all cited papers obtained good results, none of them reached the lignin removal achieved in this work.
Moniruzzaman and Ono [46] applying commercial enzymes from Trametes sp. were able to remove from 10.2% to 50.1% of the lignin of wood biomass, depending on the treatment used. Mukhopadhyay et al. [47] achieved high levels (over 80%) delignification of Ricinus comunis by applying commercial laccase in an optimization experiment (RSM). Reaction time varied from 4 to 8 hours, similar to the findings in this study, where there was no significant difference between the three different times of reaction (4, 12 and 72 hours).
The results found in this work indicate that the crude enzyme extract obtained under the optimized conditions for production of MnP by Trametes villosa (Sw.) Kreisel CCMB 651 is a possible alternative to promote the lignin removal in plant wastes. The process of enzymatic delignification may lead to better results when using optimized reaction conditions, such as time, temperature, enzyme concentration, stirring, and type of residues, which can be determined in further studies.
4. Conclusions
The optimization of culture conditions on a solid substrate for MnP secretion was performed in this study. After optimization, the maximum activity was 117.327 U/L, which was approximately 20 times greater than values reported by Machado et al. (2005) for three strains of Trametes villosa grown under submerged fermentation conditions in medium containing sugarcane bagasse as the substrate. The optimal conditions for enzyme production for sugar cane bagasse were as follows: 80% moisture content at pH 9.38 incubated at 20˚C for 15 days. The results presented here show that Trametes villosa (Sw.) Kreisel CCMB 651 has a high ligninolytic potential specially because of its MnP production. Furthermore, it was possible to cultivate the fungus by use of inexpensive agroindustrial residues, such as sugarcane bagasse.
The preliminary delignification tests showed that the crude enzymatic extract containing MnP from Trametes villosa (Sw.) Kreisel CCMB 651 was capable of reducing the lignin content by 35.05 ± 1.45 (%) for the sugar cane bagasse; 63.11 ± 0.06 (%) for the sisal fiber and 39.61 ± 0.39 (%) for the coconut shell. These results are encouraging and further studies are currently being performed to optimize the reaction conditions in order to improve the lignin removal in these plant residues and others commonly found in Northeastern Brazil. It will be possible to conduct experiments of enzymatic saccharification of cellulosic material for ethanol production from the optimized conditions.
Acknowledgements
The authors would like to thank CAPES for the scholarship.
NOTES
*Corresponding author.