The Analysis of the Quenching Efficiency of Humic Acid Fluorescence by Cadmium and Copper Ions ()
1. Introduction
Humic acids (HAs), regardless of their origin, are high nitrogen-containing organic acid molecules that contain aromatic groups. The analysis of the results obtained by a number of direct and indirect methods is allowed to create an overall model of the structure of macromolecules of HA: its core comprises aromatic carbon skeleton and the periphery contains polysaccharide-polypeptide chains [1] . The studies of the conformational properties of HAs are highly relevant because HAs play a crucial role in the formation of agriculturally important soil structure and the structure of HA largely determines its physical and chemical properties. In addition, the relevance of studying of the conformational properties of HA is due to their ability to reduce the effect of toxicants as the latter bind into HA complexes.
A characteristic structural feature of macromolecules of HAs is their variability and polydispersity of their elemental and molecular composition [1] . To assess the degree of polydispersity of HAs the numerous physical- chemical methods were used [1] - [5] , and in particular, fluorescent approaches [6] - [13] . Earlier [12] , we proposed an original method for assessing the degree of polydispersity of the sample HA. This method is based on the excitation of the fluorescence by monochromatic illumination. This technique was able to detect the spectral dependence of the fluorescence quenching efficiency. The possibility of such approach has been demonstrated by fluorescence quenching of the standard sample HA by Cu2+ ion. In this experiment, monotonic spectral dependence of the quenching constants was not received and results in the conclusion were made available at least three spectral components.
The aim of this work was to compare the spectral dependence of the efficiency of fluorescence quenching the HA samples by Cd2+ and Cu2+ ions. These ions have different radii: 0.108 nm and 0.08 nm for Cd2+ and Cu2+, respectively [14] . In this regard, it can be expected that their interaction with sites containing fluorophores will be different. This difference may result from the differences in spatial accessibility of the quencher to the site containing the fluorophores (at the same value of Van der Vaals interaction) or from the differences in magnitude of Van der Vaals interaction (at the same available space). As a result of the experiments, it was found that the spectral dependence of the fluorescence quenching efficiency of Cd2+ ion, HA, undergoes larger changes than the spectral dependence of the fluorescence quenching efficiency of HA by ion Cu2+.
2. Materials and Methods
2.1. Materials
As a sample, HA the standard preparation (Humic Asid Standard IHSS Elliot soil 1S102H) was used. We used alkaline solutions (NaOH, “Fluka”). It was used as the quencher cadmium nitrate Cd(NO3)2∙5H2O and copper sulfate CuSO4∙5H2O (“SCHDA” without further purification) were used in the experiments.
2.2. Preparation of HA Solutions
Stock solutions were prepared by dissolving HA 2 mg of sample in 20 ml of alkali solution. Solutions of lower concentrations were prepared by diluting this solution. The shaking of the initial solutions was performed for 60 minutes at room temperature followed by filtration. Measurement of the absorption spectra of the filtered and not filtered source HA solutions have shown their coincidence. Fluorescence spectra were recorded for 24 hours after the preparation of solutions.
Solutions of sulfuric and nitric acids were prepared using deionized water. When preparing solutions the scales “Sartorius” was used. The pH was ~13. pH values were measured with a pH meter “Redelkis”. All experiments were performed at (22 ± 1)˚C.
2.3. Fluorescence Equipment
The absorption spectra were obtained on a Hewlett Packard 6041 spectrophotometer. Quartz cuvettes with optical path length of 1 cm were used. Fluorescence spectra were obtained N2-pulsed laser fluorometer (λexc = 337.1 nm, illumination pulse frequency was 25 Hz, the average power of 80 mW) [12] [13] . The laser beam passed through the cell parallel to the top of the entrance slit of a fluorometer. The diameter of the laser beam was 3 mm. Distance from the center of the laser beam to the wall of the working of the cell through which the fluorescence was recorded was 0.5 cm. Cuvette was filled up with a solution to avoid formation of meniscus. Stability control of the intensity of the exciting light was conducted by periodic removal of the external standard (the intensity of Raman lines OH vibration of pure water).
2.4. Processing of the Experimental Data
Integral efficiency of fluorescence quenching was determined by the value of Stern-Volmer quenching constants 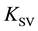 [15] from the relation
[15] from the relation
 (1)
(1)
In (1), I0 and I―the corrected integral fluorescence intensity with HA  and
and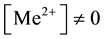 , re-
, re-
spectively ( ―cadmium or copper ion). The magnitude of the corrected integrated intensity I determined from the equation (2)
―cadmium or copper ion). The magnitude of the corrected integrated intensity I determined from the equation (2)
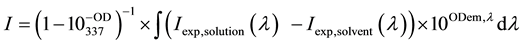 (2)
(2)
In (2), 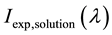 and
and 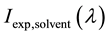 experimentally observed fluorescence intensity of solutions and sol- vent by fluorescence wavelength
experimentally observed fluorescence intensity of solutions and sol- vent by fluorescence wavelength  respectively. OD337―optical density at
respectively. OD337―optical density at .
.![]() ―optical density at the wavelength of fluorescence
―optical density at the wavelength of fluorescence ![]() at optical path length of 0.5 cm in the spectral range 350 - 650
at optical path length of 0.5 cm in the spectral range 350 - 650
nm. Factors ![]() and
and ![]() are corrective amendments on the inner filter effect in the excitation and emission of fluorescence, respectively. Integration of the modified loop fluorescence spectrum was conducted in the spectral range from 350 to 650 nm (the values of the lower and upper limit for ∫ sign).
are corrective amendments on the inner filter effect in the excitation and emission of fluorescence, respectively. Integration of the modified loop fluorescence spectrum was conducted in the spectral range from 350 to 650 nm (the values of the lower and upper limit for ∫ sign).
Typically, the efficiency of fluorescence quenching at a specific wavelength ![]() quantitatively determined by the rate constants of quenching Stern-Volmer
quantitatively determined by the rate constants of quenching Stern-Volmer ![]() from the relation [15]
from the relation [15]
![]() (3)
(3)
In (3), ![]() and
and![]() ―HA fluorescence intensity wavelength at
―HA fluorescence intensity wavelength at ![]() for
for ![]() and ≠ 0, respectively.
and ≠ 0, respectively. ![]() value was determined by the formula
value was determined by the formula
![]() (4)
(4)
However, in [13] it was shown that the magnitude ![]() cannot always be obtained from the experimental data due to the fact that in conventional rectifiability anamorphoses Stern-Volmer may be missing. In this regard, in [13] to estimate the spectral efficiency of quenching was proposed to use a parameter
cannot always be obtained from the experimental data due to the fact that in conventional rectifiability anamorphoses Stern-Volmer may be missing. In this regard, in [13] to estimate the spectral efficiency of quenching was proposed to use a parameter
![]() (5)
(5)
Such a definition of quenching efficiency means that if ![]() holds fluorescence quenching, while
holds fluorescence quenching, while ![]() the fluorescence intensity increases. In our work for the analysis of spectral efficiency of HA fluorescence quenching we also use this option.
the fluorescence intensity increases. In our work for the analysis of spectral efficiency of HA fluorescence quenching we also use this option. ![]() parameter characterizes the change in shape of the contour fluorescence upon addition of quencher and is a universal, i.e. it can be used for analysis of the spectra in all experiments. It is
parameter characterizes the change in shape of the contour fluorescence upon addition of quencher and is a universal, i.e. it can be used for analysis of the spectra in all experiments. It is
easy to see that, since ![]() (from (3)) and because any manifestation of non-monotony in
(from (3)) and because any manifestation of non-monotony in
the spectral dependence ![]() manifest in the spectral dependence of the parameter
manifest in the spectral dependence of the parameter![]() . An additional advantage of the parameter
. An additional advantage of the parameter ![]() is that the analysis of spectral dependence of the quenching efficiency cannot ignore
is that the analysis of spectral dependence of the quenching efficiency cannot ignore
the correction factor ![]() because calculation of the quantum yield is not required. Nevertheless, the
because calculation of the quantum yield is not required. Nevertheless, the
determination of the ![]() (not
(not![]() ) is crucial because it can help to evaluate the rate constant for quenching fluorescence Kq, which allows to draw conclusions about static or diffusion mechanism of fluorescence quenching [15] .
) is crucial because it can help to evaluate the rate constant for quenching fluorescence Kq, which allows to draw conclusions about static or diffusion mechanism of fluorescence quenching [15] .
In addition, the analysis of experimental data, we used the ![]() parameter―the ratio of the
parameter―the ratio of the ![]() values for Cu2+ and Cd2+ ions, respectively:
values for Cu2+ and Cd2+ ions, respectively:
![]() (6)
(6)
From the definition of this value, it implies that for ![]() copper ion more effectively quenches the fluorescence than cadmium ion, while
copper ion more effectively quenches the fluorescence than cadmium ion, while ![]() less efficiently.
less efficiently.
Fluorescence quenching rate constant ![]() [15] evaluated the relation
[15] evaluated the relation
![]() (7)
(7)
In (7),![]() ―fluorescence lifetime of the HA macromolecule in the absence of quencher. The
―fluorescence lifetime of the HA macromolecule in the absence of quencher. The ![]() values of HA samples (~3 ns) was taken from [10] .
values of HA samples (~3 ns) was taken from [10] .
3. Results
The observed absorption spectra of these HA solutions were similar to obtained earlier in [12] . The execution of the Bouguer-Lambert-Beer law was satisfactory (data not shown).
Figure 1(a) and Figure 1(b) show the experimentally observed fluorescence spectra for the systems studied. In the inset, the Stern-Volmer dependences were showed, as amended by (2). It is visible good rectifiable. Evaluation of the constants ![]() gives values (2560 ± 250) М−1 and (4300 ± 250) M−1 for quenching ions Cd2+ and Cu2+, respectively. Quenching rate constants
gives values (2560 ± 250) М−1 and (4300 ± 250) M−1 for quenching ions Cd2+ and Cu2+, respectively. Quenching rate constants ![]() were
were ![]() and
and ![]() for quenching ions Cd2+ and Cu2+, respectively. These values exceed the diffusion constants, which for aqueous solutions should be around 1010 M−1s−1 [15] . Exceeding the observed quenching rate constants of diffusion values indicates the presence of static quenching, i.e. in the studied solutions the complex formation between HA macromolecules and metal ion takes place in the dark.
for quenching ions Cd2+ and Cu2+, respectively. These values exceed the diffusion constants, which for aqueous solutions should be around 1010 M−1s−1 [15] . Exceeding the observed quenching rate constants of diffusion values indicates the presence of static quenching, i.e. in the studied solutions the complex formation between HA macromolecules and metal ion takes place in the dark.
![]()
Figure 1. The experimentally observed fluorescence spectra of HA. [Cd2+] = 0 (1); 2.5 × 10−4 M (2); 5 × 10−4 M (3); 1.1 × 10−3 M (4)-a; [Cu2+] = 0 (1); 1.25 × 10−3 M (2); 2.5 × 10−3 M (3); 3.75 × 10−3 M (4)-b. The insets: the Stern-Volmer dependences constructed according to the Equation (2).
Figure 2(a) shows the spectral dependence of ![]() for fluorescence quenching HA by ions Cd2+ (1) and Cu2+ (2). As can be seen from the figure, there is a noticeable difference in the spectral dependence of
for fluorescence quenching HA by ions Cd2+ (1) and Cu2+ (2). As can be seen from the figure, there is a noticeable difference in the spectral dependence of ![]() in the spectral range
in the spectral range ![]() nm cadmium ion quenches the fluorescence is weaker than the copper ion (
nm cadmium ion quenches the fluorescence is weaker than the copper ion (![]() values for Cd2+ ion is less than for Cu2+ ion). This means that in the macromolecule IHSS the sites contained identical fluorophores interact weakly with the cadmium ion than with the copper ion. Noteworthy is also the fact that the observed value
values for Cd2+ ion is less than for Cu2+ ion). This means that in the macromolecule IHSS the sites contained identical fluorophores interact weakly with the cadmium ion than with the copper ion. Noteworthy is also the fact that the observed value ![]() for cadmium ion at
for cadmium ion at ![]() nm, which means the increase of fluorescence intensity.
nm, which means the increase of fluorescence intensity.
From of the data of Figure 2(a) the difference in the magnitude of changes in the efficiency of fluorescence quenching ![]() of copper and cadmium ions in the spectral range of 400 - 600 nm is also very noticeable:
of copper and cadmium ions in the spectral range of 400 - 600 nm is also very noticeable:
![]() (8)
(8)
For cadmium ion value ![]() but for the copper ion
but for the copper ion ![]() Thus, the difference of values is more than 2 times. This means that the interaction of cadmium ions with sites of HA macromolecule undergoes larger changes than the interaction of copper ions with the same sites. Thus, the availability of sites for HA fluorescence quenching for cadmium ions varies more than for copper ions.
Thus, the difference of values is more than 2 times. This means that the interaction of cadmium ions with sites of HA macromolecule undergoes larger changes than the interaction of copper ions with the same sites. Thus, the availability of sites for HA fluorescence quenching for cadmium ions varies more than for copper ions.
In Figure 2(b), the spectral dependence of the ![]() values is shown. In this case, the data clearly show that the dependence of the
values is shown. In this case, the data clearly show that the dependence of the ![]() on
on ![]() is a function. The value increases with the increasing wavelength of HA fluorescence spectra. The form of the spectral dependence of
is a function. The value increases with the increasing wavelength of HA fluorescence spectra. The form of the spectral dependence of ![]() shows that upon increase of the “red” region of the spectrum fluorescence of the HA chromophore, the quenching efficiency of copper ion becomes higher.
shows that upon increase of the “red” region of the spectrum fluorescence of the HA chromophore, the quenching efficiency of copper ion becomes higher.
![]()
Figure 2. Spectral dependence of the αλ parameter. (a) For copper ion―1; for cadmium ion―2. (b) Spectral dependence of the βλ para- meter.
4. Discussion
We obtained the following facts: First, in the spectral range 400 - 600 nm cadmium ion quenches the fluorescence weaker than the copper ion; second, in the spectral range 400 - 600 nm for the cadmium ion value ![]() is greater than for the copper ion; third, in the “blue” part of the spectrum of HA fluorescence the effective quenching by ions of both metals takes place; fourth; HA fluorescence quenching increases at λ > 600 nm upon adding cadmium ion; fifth, continuous growth values
is greater than for the copper ion; third, in the “blue” part of the spectrum of HA fluorescence the effective quenching by ions of both metals takes place; fourth; HA fluorescence quenching increases at λ > 600 nm upon adding cadmium ion; fifth, continuous growth values ![]() with increasing
with increasing ![]() was observed.
was observed.
1) The decrease of the effective quenching of cadmium ion can be explained by the low access to HA fluorophores by this ion due to its larger size compared to copper ion and, consequently, by the lower efficiency of forming the complex.
2) For all sites of HA with fluorophores emitting light in the range of 400 - 600 nm, the availability of the copper ion is the same, while for the cadmium ion takes place a limit of availability.
3) Based on the proposed model, the effective quenching of ions of both metals in the “blue” part of the spectrum should be attributed to the fact that the chromophores emitting “blue” fluorescence are most accessible (not screened) and located, respectively, on the periphery of the HA macromolecule structure.
4) The increase of fluorescence intensity upon addition of cadmium ion ![]() can be interpreted by the influence of the metal ion complex formation with the macromolecule HA and structure of the macro-mo- lecule is changed partially. This result is a site in which the fluorophore undergoes complexation, which, in turn, increases its intensity. The increase in the fluorescence intensity upon the formation of complexes has been observed previously [15] .
can be interpreted by the influence of the metal ion complex formation with the macromolecule HA and structure of the macro-mo- lecule is changed partially. This result is a site in which the fluorophore undergoes complexation, which, in turn, increases its intensity. The increase in the fluorescence intensity upon the formation of complexes has been observed previously [15] .
5) Increasing quantity of ![]() with growing of
with growing of ![]() can be associated with the fact that binding of copper ions with specific sites of HA macromolecule takes place more effectively than cadmium ion in the case of sites having “red” fluorophores. Hence, the availability of sites having “red” fluorophores is higher for the smaller radius ion. This conclusion suggests that the “red” fluorophores have limited availability while availability of fluorophores causing “blue” region of the fluorescence spectrum of HA is much higher. In connection with this, the conservative assumption can be made that the “red” part of the fluorescence spectrum of HA contributes to the fluorescence of fluorophores located at the core sites of HA.
can be associated with the fact that binding of copper ions with specific sites of HA macromolecule takes place more effectively than cadmium ion in the case of sites having “red” fluorophores. Hence, the availability of sites having “red” fluorophores is higher for the smaller radius ion. This conclusion suggests that the “red” fluorophores have limited availability while availability of fluorophores causing “blue” region of the fluorescence spectrum of HA is much higher. In connection with this, the conservative assumption can be made that the “red” part of the fluorescence spectrum of HA contributes to the fluorescence of fluorophores located at the core sites of HA.
It is not difficult to see that the above explanation of the spectral dependences of ![]() is based on the assumption that the quenching efficiency depends only on the availability of fluorophores in HA structure. (The “chemical” factor was considered constant, e.g., the Coulomb interaction between the ions of copper and cad- mium, with the same accessibility site is different due to different distances between the centers of interaction.). Currently, however, to give preference to the structural and chemical nature of the origin of the spectral de- pendence of quenching efficiency is not possible at this stage of knowledge.
is based on the assumption that the quenching efficiency depends only on the availability of fluorophores in HA structure. (The “chemical” factor was considered constant, e.g., the Coulomb interaction between the ions of copper and cad- mium, with the same accessibility site is different due to different distances between the centers of interaction.). Currently, however, to give preference to the structural and chemical nature of the origin of the spectral de- pendence of quenching efficiency is not possible at this stage of knowledge.