Purification and Characterization of Glucose-6-Phosphate Dehydrogenase from Pigeon Pea (Cajanus cajan) Seeds ()
1. Introduction
Glucose-6-phosphate dehydrogenase (G6PD; D-glucose-6-phosphate: NADP+ 1-oxidoreductase; EC 1.1.1.49) is the first enzyme of pentose phosphate pathway and was first described by Warburg and Christian in 1931 [1] . It catalyzes the transformation of glucose-6-phosphate to 6-phosphogluconolactone concomitant with conversion of NADP to NADPH. Due to its involvement in various haemolytic disorders, alteration of its activity under various nutritional and hormonal conditions and its potential as a regulator for the availability of reduced NADPH, this enzyme gained considerable attraction of scientific community [2] -[7] . NADPH produced is nec- essary for the reductive biosynthesis of fatty acids, isoprenoids and aromatic amino acids in the dark, for nitro- gen assimilation in heterotrophic tissues and acts as cofactor for other antioxidative enzymes like glutathione reductase [6] -[12] . The NADPH and pentose phosphates produced also serves as the route of entry of 3 - 5 car- bon sugars to the glycolytic pathway [13] .
The enzyme is widely distributed and has been isolated from microorganisms, plants and various mammalian tissues [13] -[18] . The first isolation of the enzyme was carried out from human erythrocytes by Yoshida and Huang [19] . Affinity chromatography (2’, 5’-ADP Sepharose 4B) first used by De Flora [20] is a common tech- nique for purification of glucose-6-phosphate dehydrogenase. The reports on purification of glucose-6-phos- phate dehydrogenase from plant sources are very less. However, some attempts have been made for the purifica- tion of enzyme from plant sources, such as from spinach leaf [21] , soybean nodules [22] , potato tuber [11] , pea leaves [23] and coriander [24] . The enzyme is remarkable for its genetic diversity and many variants produced due to missense mutation have been described with wide ranging levels of enzyme activity and associated clini- cal symptoms. Glucose-6-phosphate dehydrogenase deficiency causes haemolytic anaemia in the presence of simple infection, ingestion of fava beans or reaction with certain medicines, antibiotics, antipyrectics and anti- malarial. Keeping in view the clinical significance of the enzyme and less studied from plant sources the present study has been aimed with purification and characterization of glucose-6-phosphate dehydrogenase from pigeon pea seeds.
2. Materials and Methods
2.1. Purification of Glucose-6-Phosphate Dehydrogenase from Pigeon Pea Seeds
100 g of pigeon pea seeds were washed thoroughly with distilled water and soaked for 12 hr in pre cooled 200 mL Tris HCl buffer (100 mM, pH 8.0) at 4˚C. The seeds were homogenized in a kitchen blender for 2.5 min and filtered through a piece of double layered muslin cloth and centrifuged for 45 min at 4˚C at 13,000 g. About 176 mL of crude extract was collected and stored at 0˚C - 4˚C in a refrigerator. Crude extract was subjected to 40% - 70% ammonium sulphate precipitation by adding solid ammonium sulphate. The solution was stirred for at least 30 min at 0˚C - 4˚C and precipitated proteins were removed by centrifugation for 45 min at 4˚C at 13,000 g. The precipitate was collected and dissolved in Tris-HCl buffer (20 mM, pH 8.0). The enzyme obtained from ammo- nium sulphate fractionation was subjected to 30% polyethylene glycol (PEG-4000) precipitation. The protein precipitate was collected by centrifuging the content at 20,000 g for 45 min at 0˚C - 4˚C. The pellet was dis- solved in Tris-HCl buffer (20 mM, pH 8.0) and is stored at 0˚C - 4˚C in refrigerator. The enzyme solution was dialyzed against pre-chilled Tris-HCl buffer (20 mM, pH 8.0) at 0˚C - 4˚C with 5 - 7 repeated change of the same buffer by using dialysis membrane (50 KDa). The dialyzed sample was loaded on Carboxymethyl cellu- lose column, equilibrated with 100 mM Tris-HCl buffer of pH 8.0. The flow rate was maintained 30 - 40 mL/hr. The enzyme was collected from the unbound fraction (2 mL each). The unbound samples were tested for activ- ity and protein. The enzymically active fractions were pooled and precipitated out at 90% saturation of ammo- nium sulphate. The proteins were collected and dissolved in 20 mM Tris-HCl buffer, pH 8.0. The enzyme solu- tion was again dialyzed against pre-chilled Tris-HCl buffer (20 mM, pH 8.0) at 0˚C - 4˚C with 5 - 7 repeated change of the same buffer. The dialyzed sample was loaded on DEAE-cellulose column, equilibrated with 100 mM Tris-HCl buffer (pH 8.0). The flow rate was maintained 30 - 40 mL/hr. The column was washed with Tris- HCl buffer pH 8.0. The enzyme was eluted with same buffer containing 0.2 M KCl. The different eluted frac- tions were tested for activity and protein. The enzymically active fractions were pooled and precipitated out at 90% saturation of ammonium sulphate. The proteins were collected and dissolved in 20 mM Tris-HCl buffer, pH 8.0 and stored at −20˚C. The purified enzyme was stable with a minor loss of activity for at least 45 days when stored at −20˚C in 20 mM Tris-HCl buffer, pH 8.0.
2.2. Enzyme Assay
The activity of Glucose-6-phosphate dehydrogenase enzyme has been assayed by determining the rate of forma- tion of NADPH at wavelength of 366 nm. The NADPH is formed as a result of oxidation of glucose-6-phos- phate to 6-phosphogluconolactone leading to reduction of NADP+ to NADPH. The reaction was started by add- ing 0.1 mL of appropriately diluted enzyme to 5.9 mL reaction mixture containing 0.2 mL glucose-6-phosphate (2 mM), 0.2 mL NADP+ (0.2 mM), 0.1 mL MgCl2 (3.33 mM) and 5.4 mL assay buffer (55 mM Tris-HCl, pH 8.0). The rate of increase in absorbance at 366 nm was noted. The enzyme activity was calculated from εNADPH value = 3.11 × 103 M−1∙cm−1 under mentioned conditions.
Enzyme Unit
One unit of enzyme activity is defined as the amount of enzyme required to transform 1 µmole NADP+ to NADPH in one minute under our specific test conditions.
2.3. UV Spectrum and A280/A260 Ratio
The UV spectrum of the purified enzyme was determined using UV-Vis spectrophotometer (Perkin-Elmer) in the wavelength range 240 - 360 nm.
2.4. Estimation of Protein
The protein was estimated by the method of Lowry et al. [25] , using bovine serum albumin as a standard protein for the calibration of Folin-Ciocalteau phenol reagent.
2.5. Polyacrylamide Gel Electrophoresis (PAGE)
Polyacrylamide gel electrophoresis in absence of any denaturing agent was carried out by the method of Laem- mli [26] .
2.6. Molecular Weight and Subunit Molecular Weight Determination
Molecular weight was determined by gel filtration method. A thick slurry of preswellen and degassed Sepharose 6B was poured in a gel filtration column (1 × 50 cm). It was equilibrated with several bed volumes of extraction buffer (Tris HCl, 100 mM, pH 8.0). The column was calibrated by loading 1 ml each of lysozyme (14.7 KDa), ova albumin (45 KDa), Bovine Serum Albumin (130 KDa) and isocitrate dehydrogenase (141 KDa). After washing the column with same buffer, 1 mL of enzyme preparation was loaded. The elution was carried out at 0˚C - 4˚C with extraction buffer (flow rate 10 ± 2 mL/hr). Molecular weight of the enzyme was obtained from the calibration curve plotted with elution volume versus log molecular weight.
Subunit molecular weight for glucose-6-phosphate dehydrogenase was obtained by comparing its electropho- retic mobility in presence of sodium dodecyl sulphate (SDS) with mobilities of some known proteins, as the method described by Weber and Osborn [27] .
2.7. Effect of Substrate and Coenzyme Concentration
Effect of substrate concentration on purified glucose-6-phosphate dehydrogenase was seen at different concen- trations of glucose-6-phosphate varying from 0.5 mM to 35 mM and at fixed concentration of NADP+ (0.2 mM). Effect of coenzyme concentration on purified glucose-6-phosphate dehydrogenase was seen at different concen- tration of NADP+ varying from 0.1 mM to 45 mM and at fixed concentration of glucose-6-phosphate (2 mM) by determining the rate of formation of NADPH at 366 nm.
2.8. Effect of pH on Km and Vmax Values of Substrate
Effect of pH on the Km and Vmax values of substrate has been investigated in the pH range 7.5 - 9.0. The sub- strate concentration was varied from 0.5 mM - 10 mM for each pH value and the activity was assayed by deter- mining the rate of formation of NADPH at wavelength of 366 nm.
2.9. Effect of Enzyme Concentration
Effect of enzyme concentration on purified glucose-6-phosphate dehydrogenase was seen by adding different volume of enzyme in the reaction mixture [0.1 mL (2.7 U) - 1 mL (27 U)].
2.10. Effect of pH
Effect of pH on purified glucose-6-phosphate dehydrogenase was seen by changing the pH of assay buffer in the range of 7.0 - 9.4.
2.11. Effect of Temperature on Enzyme Stability
Effect of temperature on purified enzyme was carried out by incubating the enzyme at different temperatures (ranging from 20˚C to 50˚C) for 5 min and then measuring the activity at 366 nm.
2.12. Thermal Inactivation
Thermal inactivation of purified NADP+ linked glucose-6-phosphate dehydrogenase from pigeon pea has been studied at different temperatures, i.e. 30˚C and 40˚C. For this experiment the enzyme solutions were incubated at 30˚C and 40˚C in water bath and aliquots were withdrawn at different time intervals and were chilled in the ice cold water and assayed for the activity of NADP+ linked glucose-6-phosphate dehydrogenase.
2.13. Substrate Specificity
Substrate specificity of purified glucose-6-phosphate dehydrogenase was observed by using different substrates such as glucose-1-phosphate, glucose, fructose and galactose-6-phosphate. The reaction was started by adding 0.1 mL of appropriately diluted enzyme to 5.9 mL reaction mixture containing 0.2 mL, 2 mM different sub- strates (glucose-1-phosphate, glucose, fructose and galactose-6-phosphate), 0.2 mL NADP+ (0.2 mM), 0.1 mL MgCl2 (3.33 mM) and 5.4 mL assay buffer (55 mM Tris-HCl, pH 8.0). The rate of increase in absorbance at 366 nm was noted.
3. Results and Discussion
3.1. Purification of Glucose-6-Phosphate Dehydrogenase from Pigeon Pea Seeds
Glucose-6-phosphate dehydrogenase was purified from pigeon pea by employing ammonium sulphate (40% - 70%) fractionation, polyethylene glycol precipitation, Carboxymethyl cellulose column chromatography and die- thylaminoethyl cellulose column chromatography. The finally purified enzyme obtained by diethylaminoethyl cellulose column chromatography was found to be 123.69 fold purified with a specific activity of 12.79 U/mg and 21.37% recovery. A sample protocol of purification and results obtained is documented in Table 1. An elu- tion profile of pigeon pea enzyme is given in Figure 1. 7.5% native Polyacrylamide gel electrophoresis yields a single protein band (Figure 2) indicating that the protein was obtained in pure form.
3.2. UV Spectrum and A280/A260 Ratio
The UV spectrum of the purified enzyme was determined using UV-Vis spectrophotometer (Perkin-Elmer). The purified glucose-6-phosphate dehydrogenase shows a typical characteristic protein absorption spectrum in ultra- violet region with maximum absorbance of 0.39 (protein concentration 2.1 mg/ml) at 280 nm. The A280/A260 ra- tio of purified enzyme was found to be 1.5 suggesting that the enzyme preparation was free from nucleotides. The absorption spectrum of pigeon pea glucose-6-phosphate dehydrogenase is shown in Figure 3.
3.3. Molecular Weight Determination
Molecular weight of pigeon pea glucose-6-phosphate dehydrogenase was determined by using gel filtration chromatography. Molecular weight of the enzyme was obtained from the calibration curve plotted with elution
![]()
Table 1. Purification table of glucose-6-phosphate dehydrogenase enzyme from 100 g pigeon pea seeds.
![]()
Figure 1. DEAE-cellulose elution profile of glucose-6-phosphate dehydrogenase of pigeon pea. The pigeon pea glucose-6-phosphate dehydrogenase was eluted with 100 mM Tris-HCl buffer containing 0.2 M KCl, pH 8.0, in different fractions tested for enzyme activity ( ) and protein ( ).
![]()
Figure 2. 7.5% native PAGE of purified NADP+ linked glucose-6-phosphate dehydrogenase visualized by coomassie brilliant blue staining. 90 μg of protein was loaded.
![]()
Figure 3. Ultraviolet absorption spectrum of purified pigeon pea glucose-6-phosphate dehydrogenase. The maximum absorbance is found at 280 nm.
volume versus log molecular weight (Figure 4). The molecular weight of purified glucose-6-phosphate dehy- drogenase was found to be 113 KDa. The molecular weight of glucose-6-phosphate dehydrogenases from hu- man erythrocyte was reported to be 105 KDa [28] , Pseudomonas W6 native enzyme was reported to be 123 ± 5 KDa [29] , isozymes of spinach was found to have a molecular weight of 105 ± 10 KDa [21] , Methylomonas M15 glucose-6-phosphate dehydrogenase was found to have a molecular weight of 108 ± 5 KDa [30] . A 112 KDa dimeric protein was reported from Schizosaccharomyces pombe [31] .
Subunit molecular weight for pigeon pea glucose-6-phosphate dehydrogenase was obtained by the method of Weber and Osborn [27] . After quantitating the relative mobility in SDS-PAGE, the molecular mass of purified glucose-6-phosphate dehydrogenase was found to be 55 KDa (Figure 5) indicating that the pigeon pea enzyme is a homodimer. The subunit molecular weight of pigeon pea glucose-6-phosphate dehydrogenase was found to be quite close to the molecular weight of enzyme obtained from Cryptococcus neoformans (50 KDa) [32] , A. vinelandii (52 KDa) [33] , dog liver (52.5 KDa) [34] , A. aculeatus (52 ± 1.1 KDa) [15] , goat erythrocyte (52 KDa) [35] . However, the value is lower than as reported from Pseudomonas W6 [29] , pig liver [20] , turkey erythrocyte [36] , coriander [24] and rainbow trout [37] . The result revealed that pigeon pea glucose-6-phosphate dehydrogenase is a homodimer with molecular weight of 113 KDa and subunit molecular weight approximately equals to 55 KDa. Active glucose-6-phosphate dehydrogenase from various sources has been reported as dimer, tetramer or hexamer except that of rainbow trout where the protein is active in its monomeric form [37] .
3.4. Effect of Substrate and Coenzyme Concentration
Effect of varying concentration of glucose-6-phosphate on the oxidation of glucose-6-phosphate was studied in Tris-HCl buffer (55 mM, pH 8.0). The substrate concentration was varied from 0.5 mM to 35 mM. The results (Figure 6) revealed that at low substrate concentration (0.5 mM to 2 mM), the increase in rate of reaction was directly proportional to the concentration of substrate. However, further increase in glucose-6-phosphate con- centration leads to insignificant increase in enzyme activity (Figure 6). In order to determine the Michaelis con- stant (Km) and maximum velocity (Vmax), the observations of Figure 6 were replotted into Lineweaver-Burk (LB) plot (Figure 7). The Km and Vmax value for glucose-6-phosphate was found to be 2.68 mM and 0.11 U/ml re- spectively. The Km value was found to be higher than other reported Km values in plant sources such as pea leaves (2000 µM) [23] , potato tuber (260 µM) [11] and coriander (116 µM) [24] . The Km value was also greater
![]()
Figure 4. Determination of molecular weight of pigeon pea glucose-6-phosphate dehydrogenase by gel filtration.
![]()
Figure 5. Determination of molecular weight of pigeon pea glucose-6-phosphate dehydrogenase. The molecular weight of purified glucose-6-phosphate dehydrogenase was estimated by comparing relative mobility of proteins of known molecular weight with that of pigeon pea glucose-6-phos- phate dehydrogenase.
![]()
Figure 6. Effect of glucose-6-phosphate concentration on pigeon pea glucose-6-phos- phate dehydrogenase activity. The activity of suitably diluted enzyme was assayed in Tris-HCl buffer (55 mM, pH 8.0) in presence of varying concentrations of glucose-6- phosphate. The experiment was carried out in triplicate and graph was drawn by taking the mean.
![]()
Figure 7. Determination of Km and Vmax for glucose-6-phosphate using Lineweaver-Burk plot. The activity of enzyme was assayed by varying glucose-6-phosphate concentration in the reaction mixture (0.5 mM to 35 mM). The experiment was carried out in triplicate and graph was drawn by taking the mean.
than reported from microbial and animal sources [15] [35] [38] . The Vmax value was found to be greater than the value reported from coriander (0.038 U/ml) [24] . The Vmax value from other plant sources has not been reported. The Vmax value of glucose-6-phosphate was less than that of reported from animal sources, such as goose eryth- rocyte (0.28 U/ml) [39] , turkey erythrocyte (0.5 U/ml) [36] and rainbow trout erythrocyte (1.352 U/ml) [37] .
The pigeon pea glucose-6-phosphate dehydrogenase shows absolute specificity towards coenzyme NADP+, whereas it shows negligible activity with NAD+. Thus the detailed kinetics experiments have been carried out with NADP+ only. The initial rate of reaction was determined at various concentrations of NADP+. The results are shown in Figure 8. The Km and Vmax for NADP+ using Lineweaver-Burk plot was found to be 0.75 mM and 0.13 U/ml which was greater than that of values reported from pea leaves (500 µM) [23] , potato (6 µM) [11] co- riander (26 µM, 0.035 U/ml) [24] , rat liver (100 µM) [40] , Acetobacter hansenii (340 µM) [41] , goose erythro- cyte (7.4 µM, 0.286 U/ml) [40] , turkey erythrocyte (17.1 µM, 0.37 U/ml) [36] and rainbow trout erythrocyte (166 µM, 0.275 U/ml) [37] . However, the Km value of NADP+ was found to be less than that of glucose-6-phos- phate which indicates that glucose-6-phosphate dehydrogenase shows more affinity towards NADP+. Similar results have been reported by various workers [11] [15] [23] [24] [35] [36] [38] -[42] . The initial rate of reaction was also determined at various concentrations of NADP+. The double reciprocal plot converges at a point above the abscissa (Figure 9) indicating a sequential binding mechanism in which both substrates must bind to enzyme simultaneously before product formation can occur. The results (Figure 9, Figure 10) depicted that the 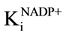 is larger than
is larger than 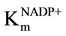 indicating that the binding of substrate enhances the affinity of the enzyme towards co- enzyme [15] .
indicating that the binding of substrate enhances the affinity of the enzyme towards co- enzyme [15] .
3.5. Effect of pH on Km and Vmax Values of Substrate
Influence of pH on the Km and Vmax values of substrate has been investigated in the pH range 7.5 - 9.0. The double reciprocal plot gives a family of lines which converges at a point on the X-axis (Figure 11). The data of Figure 11 shows that the activity of pigeon pea glucose-6-phosphate dehydrogenase decreases as pH is lowered. Thus, suggests that at pH below 8.5, proton behaves as “non-competitive inhibitor”. The pKa value was found to be 10.41, indicating that the amino acid residue at the active site might be lysine.
![]()
Figure 8. Determination of Km and Vmax for NADP+ using lineweaver-burk plot. The activity of enzyme was assayed by varying NADP+ concentration in the reac- tion mixture (0.1 mM to 45 mM). The experiment was carried out in triplicate and graph was drawn by taking the mean.
![]()
Figure 9. Double reciprocal plot for the action of NADP+ linked glucose-6-phosphate dehydrogenase of pigeon pea. Initial velocity was determined at various fixed concentrations of NADP+ with respect to varying concentration of glucose-6-phosphate at pH 8.0 in assay buffer. The experiment was carried out in triplicate and graph was drawn by taking the mean.
![]()
Figure 10. Secondary plot of Slope1/Glucose-6-phosphate versus 1/NADP+ for the purified NADP+ linked glucose-6-phosphate dehydrogenase for determination of . The experiment was carried out in triplicate and graph was drawn by taking the mean.
. The experiment was carried out in triplicate and graph was drawn by taking the mean.
![]()
Figure 11. Study of effect of pH variation on Km and Vmax values of glucose-6-phos- phate for the pigeon pea NADP+ linked glucose-6-phosphate dehydrogenase enzyme. The pH of the assay system was varied in the range of 7.5 - 9.0 with respect to the different fixed concentration of substrate. The activity was expressed in terms of rate of change of absorbance per minute at 366 nm. The experiment was carried out in triplicate and graph was drawn by taking the mean.
3.6. Effect of Enzyme Concentration
Effect of enzyme concentration on purified pigeon pea glucose-6-phosphate dehydrogenase was seen by adding different volumes of enzyme in the reaction mixture. The rate of formation of NADPH increases with increasing enzyme concentration (Figure 12).
3.7. Effect of pH
The effect of pH on glucose-6-phosphate dehydrogenase activity was examined using Tris-HCl buffer (55 mM) of varying pH values ranging from 7.0 to 9.4. The result (Figure 13) shows that the pigeon pea glucose-6- phosphate dehydrogenase has a pH optimum of 8.2. The pH optima is in accordance to that reported from Neu- rospora crassa (pH 7.4 - 8.2) [19] and very close to that of pea leaves (pH 8.0) [23] . However, pH optimum is less as compared to two isozymes of spinach (pH 9.0 and 9.2) [21] .
3.8. Effect of Temperature on Enzyme Stability
Effect of temperature on pigeon pea glucose-6-phosphate dehydrogenase was carried out by incubating the en- zyme solution in a temperature range of 20˚C - 50˚C for 5 minutes (Figure 14). The result revealed that pigeon pea glucose-6-phosphate dehydrogenase is highly thermosenstive and the activity of the soluble enzyme starts decreasing after 30˚C which might be due to thermal denaturation of the enzyme above 30˚C. Optimum tem- perature of glucose-6-phosphate dehydrogenase from coriander and D. radiophilus was found to be 30˚C [24] [43] . The activities of the two isoforms of D. radiophilus decrease above 40˚C [43] .
3.9. Thermal Inactivation
Thermal inactivation studies of purified NADP+ linked glucose-6-phosphate dehydrogenase from pigeon pea
![]()
Figure 12. Effect of enzyme concentration on pigeon pea glucose-6-phosphate dehydrogenase activity. The activity of enzyme was assayed in Tris-HCl buffer (55 mM, pH 8.0) by adding increasing volume of enzyme. The experiment was carried out in triplicate and graph was drawn by taking the mean.
![]()
Figure 13. Effect of pH on pigeon pea glucose-6-phosphate dehydrogenase activity. The activity of enzyme was assayed in Tris-HCl buffer (55 mM) by varying pH values ranging from 7.0 to 9.4. The experiment was carried out in triplicate and graph was drawn by taking the mean.
revealed that there is a progressive inactivation of enzyme with time at different temperatures. Semilog plot of the thermal inactivation (Figure 15) of enzyme was found to be linear, indicating that the thermal inactivation of pigeon pea enzyme follows simple first order kinetics at 30˚C and 40˚C with half life of 6 and 1.5 minutes re- spectively.
![]()
Figure 14. Effect of temperature on pigeon pea glucose-6-phosphate dehydrogenase activity. The enzyme was incubated at different temperatures ranging from 20˚C - 50˚C for 5 minutes and then the activity was measured at 366 nm. The experiment was carried out in triplicate and graph was drawn by taking the mean.
![]()
Figure 15. Kinetics of thermal inactivation of NADP+ linked glucose-6-phos- phate dehydrogenase at different temperatures. The enzyme solutions were incubated at 30˚C and 40˚C. The aliquots were withdrawn at different intervals of time and assayed immediately for the activity of enzyme at 366 nm. The experiment was carried out in triplicate and graph was drawn by taking the mean.
3.10. Substrate Specificity
To determine the substrate specificity of enzyme, glucose-1-phosphate, glucose, fructose and galactose-6- phosphate were used as substrates. Out of these only galactose-6-phosphate was found to be oxidized by glu- cose-6-phosphate dehydrogenase. However, the relative rate of oxidation was low. The Lineweaver-Burk plot of galactose-6-phosphate (Figure 16) shows higher Km value (Km = 3.23mM) and lower Vmax value (Vmax =
![]()
Figure 16. Determination of Km and Vmax for galactose-6-phosphate using Lineweaver- Burk plot. The activity of enzyme was assayed by varying galactose-6-phosphate concentration in the reaction mixture (0.5 mM to 35 mM). The experiment was carried out in triplicate and graph was drawn by taking the mean.
0.008879 U/ml) than glucose-6-phosphate. The higher Km value directly indicates that glucose-6-phosphate de- hydrogenase have lower affinity towards galactose-6-phosphate. Similar results were reported in case of Neuro- spora crassa [19] , however, in case of human placental enzyme affinity towards glucose-6-phosphate and ga- lactose-6-phosphate was found to be nearly equal [44] .
4. Conclusion
Glucose-6-phosphate dehydrogenase has been extracted from pigeon pea seeds and was purified to about 123.69 fold with a specific activity of 12.79 U/mg and 21.37% recovery. Molecular weight and subunit molecular weight of enzyme was found to be 113 KDa and 55 KDa respectively. The purified enzyme exhibited maximum activity at pH 8.2. Thermal stability studies showed that enzyme is quite heat sensitive and gets denatured at temperature above 30˚C. The enzyme shows absolute specificity towards coenzyme NADP+. The results indi- cate a sequential binding mechanism in which both substrates must bind to enzyme simultaneously before prod- uct formation can occur. The pKa value was found to be 10.41 indicating that lysine might be present at the ac- tive site of the enzyme. The enzyme is of great clinical importance as it is involved in various haemolytic disor- ders and is a potential regulator of reduced NADPH which is required for various biosynthetic processes. The immobilized form of enzyme has been used for various purposes such as for treatment of jaundice, as biosensors, analysis of ATP etc., hence a detailed study on the regulation and immobilization of enzyme will be of great importance.
Acknowledgements
Authors are grateful to University Grants Commission (UGC) for financial support in the form of research scholarship to Siddhartha Singh at Department of Biochemistry, Faculty of Science, Banaras Hindu University.
NOTES
*Corresponding author.