Effects of Zinc and Ascorbic Acid Application on the Growth and Photosynthetic Pigments of Millet Plants Grown under Different Salinity ()
1. Introduction
Soil salinity is a major abiotic stress for crop production in many parts of the world. Approximately one third of the irrigated area in the world (227 million hectares) is already affected by varying degree of excess salinity/sodicity [1] , primarily caused by inadequate drainage. About 23% and 37% of the world’s cultivated lands (1.5 × 109 ha) are characterized as saline and sodic, respectively.
The negative effects of salinity on plant growth and metabolism were reported on millet [2] , wheat [3] -[5] , barley [6] [7] , and rice [1] .
Zinc (Zn) is required for plant growth as an activator of several enzymes and is directly involved in the biosynthesis of growth regulators such as auxin, which promotes production of more plant cells and biomass that will be stored in the plant organs especially in seeds [8] .
Ascorbic acid is an organic acid with antioxidant properties. Many oxidants, typically reactive oxygen species such as the hydroxyl radical (formed from hydrogen peroxide), contain an unpaired electron and thus are highly reactive and damage plant cells at molecular level. This is due to their interaction with nucleic acid, proteins, and lipids [9] .
The problems of salinity can be mitigated by developing crop cultivars with improved tolerance to salts and/ or by altering the growth and physiology of the crop by ways of amendments such as fertilizers and/or phyto- chemicals [10] -[12] . The objective of this study was to evaluate the potential for mitigation of salt stress in millet plants by foliar application of Zn sulfate with or without combination of ascorbic acid.
2. Materials and Methods
A pot experiment was conducted in a greenhouse at the National Research Centre (NRC), Dokki, Cairo, Egypt, during 2005 summer season. A clay soil was sampled (0 - 15 cm depth) from Kirdasa village, Giza governorate. The bulk soil was air-dried, sieved to pass 2 mm sieve and five replicate samples were taken for analyses of some basic physiochemical properties (Table 1). Metallic pots (35 cm diameter, and 50 cm depth) were used with 30 kg air-dried soil. The inner surface of the pots was coated with three layers of bitumen to prevent direct contact between the soil and the metal. The base of the pot was filled with 2 kg gravel (about 2 - 3 cm diameter) prior to filling the pots with soil.
Each pot received 3 g calcium super phosphate (6.8% P) and 1.5 g potassium sulfate (40.3% K) and mixed with top 10 cm depth soil. These rates were equivalent to 106 and 212 kg/ha P and K, respectively. The soil was moistened to field capacity (22% water by weight) prior to planting. Millet (Pennisetum glaucum (L.) R. Br.) seeds were sown (10 seeds/plot) on July, 5, 2005. The plants were thinned 15 and 25 days after seedling emergence to leave three uniform plants/pot. Nitrogen was broadcasted using ammonium sulfate (20.5% N), 6.86 g per pot [equivalent to 488 kg・N・ha−1] in two equal doses (244 kg・ha−1 each) i.e. before planting and 2 weeks after the seedling emergence. The treatments included:
1) Main Treatments: 3 levels of irrigation water quality, i.e. tap water and 2 dilute sea water with salinity levels of 250, 2500, and 5000 ppm, respectively (i.e. 0.39, 3.9, and 7.8 dS・m−1).
2) Sub treatments: Two foliar sprays (21 and 36 days after sowing), using: i) tap water as Control; ii) Ascorbic acid (150 ppm); iii) as in treatment ii) plus 200 ppm zinc sulphate.
![]()
Table 1. Physical and chemical analysis of the soil used in this experiment.
Soil chemical analysis
High salinity water irrigation began 30 days after sowing. Each high salinity water irrigation was alternated by tap water irrigation through the entire duration of the experiment.
Each of the above 9 treatments were replicated 6 times. Thus the total number of pots was: 3 × 3 × 6 = 54. The mean minimum and maximum temperatures during the course of the experiment were 22˚C and 37˚C, respectively. Relative humidity range was 51% - 64%. The range of day length was 11 to 14 h.
On 45 days after the seedling emergence, five leaves were sampled per plant from two plants per pot for analyses of photosynthetic pigments i.e. chlorophyll_a (Chl_a), chlorophyll_b (Chl_b) and total carotenoids concentrations using the procedures described by von Wetestien et al. [13] . Plants from all replicate pots of all treatments were cut 2 cm above the soil surface. The plant height was measured by a ruler. Number of leaves was counted. The stem diameter was measured using a caliper. Leaves were separated from the stem, and leaf area was measured using a Li-Cor portable leaf area meter. The fresh biomass weights of leaves and stem were measured. The biomass of leaves and stem were dried in an oven at 72˚C for 48 h and the dry weights were recorded.
The significance of the treatments effects on the response parameters was evaluated by analysis of variance (ANOVA) and mean separation tests [14] .
3. Results and Discussion
Increased salinity in irrigation water decreased the growth of millet plants (Table 2). Significant decrease in growth parameters occurred at the high salinity level as compared to the growth of the plants irrigated by tap water. The stem or total plant dry weights decreased by 52% in plants irrigated with 7.8 dS・m−1 salinity water as compared to those of the plants irrigated by tap water. These results are in agreement with those of other researchers [12] [15] -[17] .
Olmos and Hellín [18] reported that adaptation of cell line of Pisumsativum germplasm to NaCl depends on modification of the osmotic adjustment together with physiological and biochemical modifications. When cultivar calli, with tolerance to high salinity, was grown in high NaCl medium (85.5 mM), intracellular levels of Na, Cl, reducing sugars were increased. Total free amino acids and ascorbic acid contents also increased. Veeranagamallaiah et al. [19] reported reduction in millet (Setariaitalica L. cv Prasad) seedling growth and biomass when subjected to 100 - 200 mM NaCl. Furthermore, Beltagi [11] reported no significant negative effects of salinity (20 and 40 mM) on stem and root length of pure strain of Chickpea. However, stem and root fresh weights and the root dry weight were significantly decreased at 40 mM NaCl. The number of leaves per plant was decreased at 20 and 40 mM NaCl.
The negative effects of salt stress were reported on water and mineral absorption [20] -[22] , water adjustment [23] [24] , protein synthesis [19] [25] , photosynthesis and carbohydrate accumulation [23] [26] , enzymes activities [27] [28] , growth regulators [7] [29] and antioxidant defense mechanism [30] [31] .
Salinity stress had no significant effects on the Chl_a and Chl_b content (Table 3). Carotenoid content, however, decreased significantly in the plants subjected to irrigation with 3.9 dS・m−1 salinity water as compared to that of the plants received tap water irrigation. Further increase in salinity to 7.8 dS・m−1 had no significant effects on the carotenoid content. The ratio of (Chl_a + Chl_b): carotenoid increased with an increase in salinity levels.
Beltaji [11] reported no negative effects of salinity on Chlorophyll content in chick pea plants. Pinheiro et al. [23] reported that Chl_a and carotenoids contents in castor bean increased with increased salinity in the range of 0 to 30 mM NaCl salinity on 38 days after germination, but decreased on 59 days after germination. Chl_b content,
![]()
Table 2. Effects of salinity on growth and biomass of millet plants.
1Tap water; 2LSD: Least significant difference; 3NS: Non-significant.
however, decreased at both stages and the reverse was true for Chl_a: Chl_b ratio. On the other hand, Sairam and Srivstava [32] reported a decrease in chlorophyll content in wheat genotypes subjected to 6.85 dS・m−1 salinity using NaCl.
4. Response to Foliar Application of Ascorbic Acid and Zinc Sulfate
Foliar application of only ascorbic acid significantly increased number of leaves and leaf area as compared to those of the plants which were sprayed with tap water (Table 4). All other response parameters were non-sig- nificant. Plant height, number of leaves, and total plant dry biomass were significantly increased with foliar application of ascorbic acid plus zinc sulfate. Foliar application of Zinc has contributed to increased plant growth and yield of peanuts [33] [34] and sunflower [35] .
Beltagi [11] indicated that the addition of ascorbic acid (4 mM) significantly increased the stem dry weight of chickpea plants. Abd El-Moniem et al. [36] reported foliar application of zinc on orange trees’ improved leaf N, K, and Zn concentrations. Abd El-Aziz et al. [37] also reported increased growth and nutrient uptake by Kaya sengalensis with foliar Zinc application.
Zinc is an important activator of several enzymes in plants and is directly involved in the biosynthesis of growth substances, such as auxin which produces more plant cells which result in increased dry matter. Darwish et al. [33] reported the highest seed and oil yields, and protein percentage of peanuts grown with foliar application of 96 kg・m−1 K. Gobarah et al. [34] also reported an increase in peanut yield and quality with foliar application of 2% zinc solution. Similar responses were also reported for sunflower [35] . No significant response was evident on photosynthetic pigments by foliar application of ascorbic acid alone or in combination with zinc sulfate (Table 5).
The interactions between the salinity and foliar application of ascorbic acid and zinc sulfate were mostly insignificant on all growth parameters, except leaf area and stem dry weight (Table 6), and photosynthetic pigments, except Chl_a + Chl_b (Table 7). A significant increase in stem dry weight of chickpea by application of ascorbic acid (4 mM) has been reported only at the low salinity level (20 mM NaCl) [11] . Beneficial effects of the exogenous application of ascorbic acid in partially mitigating the adverse effects of salt stress on growth of Chickpea plants (Cicer arietinum L.), cell division and cell enlargement have been reported [11] [38] . Shalata and Neumann [39] reported that salt-stress increased the accumulation of lipid peroxidation products produced
![]()
Table 3. Effects of salinity on the concentrations of photosynthetic pigments of millet plants.
LSD: Least significant difference; NS: Non-significant; Chl_a: Cholorphyll_a; Chl_b: Cholorphyll_b.
![]()
Table 4. Effects of foliar application of ascorbic acid without or with zinc sulfate (ZnSO4) on growth and biomass production of millet plants.
LSD: Least significant difference; NS: Non-significant.
![]()
Table 5. Effects of foliar application of ascorbic acid with or without zinc sulfate on the concentrations of photosynthetic pigments in millet plants.
LSD: Least significant difference; NS: Non-significant; Chl_a: Cholorphyll_a; Chl_b: Cholorphyll_b.
![]()
Table 6. Effects of foliar application of ascorbic acid and zinc sulfate on growth of millet plants under different salinity levels.
LSD: Least significant difference; NS: Non-significant.
![]()
Table 7. Effects of foliar application of ascorbic acid and zinc sulfate on concentrations of photosynthetic pigments in millet plants grown under different salinity levels.
LSD: Least significant difference; NS: Non-significant.
by interactions with damaging active oxygen species in roots, stems and leaves. Exogenous application of ascorbic acid partially mitigated the above response, but did not significantly reduce sodium uptake or plasma membrane leakiness. Verma and Mishra [40] reported that salinity caused reduction in seedling growth and biomass accumulation, which was parallel to that caused by increased superoxide (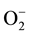 ), hydrogen peroxide (H2O2) levels, lipid peroxidation and electrolyte leakage in leaf tissues.
), hydrogen peroxide (H2O2) levels, lipid peroxidation and electrolyte leakage in leaf tissues.
Beltagi [41] reported that application of ascorbic acid (4 mM) increased Chl_a content in cowpea plants under high salinity conditions. However, Chl_b and Chl_a + Chl_b contents were not influenced by exogenous ascorbic acid. Salt stress can lead to oxidative stress through an increase in reactive oxygen species which are highly reactive and may cause cellular damage. Ascorbic acid acts as an antioxidant for scavenging hydrogen peroxide [41] . Sairam and Srivastava [32] revealed that NaCl salinity caused decrease in relative water content (RWC), and chlorophyll content. Results of this study demonstrate that salinity-induced growth suppression of millet plants can be mitigated by foliar application of ascorbic acid in combination with zinc sulfate.