Controlled-Release Analysis of Potassium Permanganate Using PMMA Matrix ()
1. Introduction
In situ chemical oxidation (ISCO) has been successfully used to degrade chlorinated hydrocarbons for decades [1] [2] . One popular oxidant in ISCO application is potassium permanganate, KMnO4 possibly due to its high oxidation potential across the pH [3] . This oxidant is highly soluble in aqueous media but largely insoluble in non-polar solvents (e.g. TCE contaminated zone). Thus, as a strong and reactive oxidant, KMnO4 would oxidize any reductive material dissolved in an aqueous medium. The amount of KMnO4 therefore available to oxidize target contaminant is likely consumed by dissolved organic constituent, thereby decreasing the oxidation efficiency. More KMnO4 than necessary is therefore used for complete degradation of the target compound [4] . Therefore, the availability of KMnO4 in aqueous phase can be controlled by slowing down its dissolution rate, thereby enabling oxidant persistence.
Controlled-release technology has widely been used in biopolymer engineering in drug delivery applications [5] -[7] . This enabled the control of dissolution rate and delivery to targeted parts due to preferential interaction. Similarly, the controlled-release application of KMnO4 in soil and water treatment should not only control the rate of oxidant dissolution but also improve effective interaction with target contaminant. This is achieved by encapsulating the water soluble KMnO4 with a responsive polymer that is not soluble in water [8] . Thus, the outer surface barrier prevents direct contact between the oxidant and the aqueous medium. Poly(methyl methacrylate) (PMMA) is hydrophobic and responsive to chlorinated hydrocarbons’ solvent environment. However, before this study PMMA has not been investigated for oxidant encapsulation.
Poly(methyl methacrylate) (PMMA) is a synthetic resin produced from the polymerization of methyl methacrylate, a transparent rigid plastic and often referred to as organic glass. PMMA, an ester of methacrylic acid (CH2 = C[CH3]CO2H), belongs to the important acrylic family of resins. In modern production it is obtained principally from propylene. Methyl methacrylate, (CH2 = C[CH3]CO2H3) in bulk liquid form or suspended as fine droplets in water, is polymerized (its molecules linked together in large numbers) under the influence of free-radical initiators to form solid PMMA. The structure of the polymer repeating unit is presented in Figure 1.
More studies would be required to understand the release mechanism of KMnO4 from PMMA encapsulation. However, this study centers on the oxidant release efficiency of PMMA in aqueous system and the matrix surface analysis.
2. Materials and Methods
2.1. Reagents
Poly (methymetacrylate) (PMMA) bead particles was ordered from Acros Organics; Sodium Borate purchased from Mallinckrodt, inc; Acetone (100%) was purchased from Acros Organics; Trichloroethylene, 100% (American Chemical Society, ACS grade), potassium permanganate (oxidant), sodium thiosulfate (used to quench reaction) and HPLC grade hexane (liquid-liquid extraction) were all purchased from Fisher Scientific. De-ionized water was used for all experiments.
2.2. Encapsulation Procedure
KMnO4 (oxidant) was encapsulated in PMMA matrix by adapting and modifying the molten suspension and cooling method at room temperature [8] . A predetermined amount (e.g. 2 g) of PMMA was measured into a 100 ml beaker and 3 ml acetone is added. The PMMA whose melting point is very high melts at room temperature under the influence of acetone. The molten PMMA is placed on a stirrer and mixed thoroughly using a glass magnetic arrow. Borax (Sodium Borate) was added to initiate cross-link of the molten PMMA. Under continuous mixing, particles of KMnO4 were added as required to produce 1:2, 1:4 and 1:8 of KMnO4 of PMMA ratio. After mixing for 10 mins to assume uniform particle dispersion, the molten mixture was allowed to solidify under room temperature by volatilizing acetone. Solidified particles/granules of encapsulated KMnO4 were reduced using laboratory mill. Sieved sizes were used as needed for the release experiments. The flow diagram (see Figure 2) depicts the encapsulation process.
![]()
Figure 1. Structure of poly methyl methacrylate.
![]()
Figure 2. Encapsulation of KMnO4 using PMMA.
2.3. Release Experiments
Encapsulated KMnO4 for control release experiment were used in the ratios of 1:2, 1:4, and 1:8 of the KMnO4/
PMMA matrix. A calculated amount of the encapsulated KMnO4 ranging from 300 to 900 mg containing equivalent amount of the oxidant were used. At ambient condition, the modified oxidant was introduced into a 1-L Milli-Q de-ionized water with continuous mechanical stirring to enable uniform solution. At specific time intervals, aliquots of the solution were taken, centrifuged and analyzed for KMnO4 using Cary UV Spectrophotometer at 525 nm wavelength. Release profiles were developed for the mass of KMnO4 released with time. The amount of KMnO4 initially contained in the PMMA matrix was calculated as below:
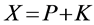 (1)
(1)
But ratio (r) of PMMA/KMnO4 can be represented as
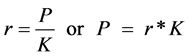 (2)
(2)
where X = weighed total mass of PMMA and KMnO4 in the encapsulated form, K = Mass of unmodified KMnO4 particles per mass of encapsulated matrix, P = mass of PMMA bead particles per mass of encapsulated matrix and r = ratio of PMMA to KMnO4 in a specific matrix.
3. Results and Discussion
3.1. Release Experiment for Encapsulated KMnO4 in Water
The release profile for KMnO4 in Water was studied with mass to mass ratios of 1:2, 1:4, and 1:8 of KMnO4 to PMMA particles respectively. The results indicate that the release efficiency is inversely proportional to the mass ratio of KMnO4 to PMMA particles. There was decrease in release with increasing mass ratio (see Figure 3). At a 10-hour time period, 79%, 55.35% and 33.59% of KMnO4 has been released from 1:2, 1:4, and 1:8 encapsulated matrix respectively. PMMA is not soluble in water hence initial release near time t = 0 is most likely dissolution of KMnO4 integrated on the surface of encapsulated matrix. Therefore the lowest release rate associated with 1:8 mass ratio was due to the thickness of the PMMA layer in the encapsulated matrix.
3.2. Analysis of Release
The release of KMnO4 from the surface of the PMMA matrix left some patches and holes under SEM imaging (see Figure 4(b)). The structural changes on glassy polymer surfaces have been attributed to possible effects of STRESS and strains on surface as the material swells [9] . This is suggested to be as a result of response to any of the following: effects of temperature change, effects of activity differences, and effects of prehistory and sample dimensions [10] -[12] . The release has therefore been modeled as non-fickian where diffusion and dissolution depends on stress on the matrix and not on concentration gradient.
![]()
Figure 3. Release profile KMnO4 from PMMA encapsulated KMnO4.
![]() (a)
(a)![]() (b)
(b)
Figure 4. (a) SEM image of the surface of PMMA encapsulated KMnO4 before release experiment; (b) SEM image of the surface of PMMA encapsulated KMnO4 after release experiment.
The initial swelling/dissolution from the matrix surface leaves patches and holes on the matrix. These holes allow the aqueous phase to diffuse into the inner opening of the PMMA matrix contacting more KMnO4 [8] . Thus the release mechanism for glassy polymers (such as polystyrene, PMMA) had previously been modeled as below [10] :
 (3)
(3)
where Se is the percent release of the oxidant, t is the time period for specific Se, constant a is the characteristic property of the encapsulated matrix and exponent n varies with parameters of structural changes. Figure 4(a) and Figure 4(b) compares respectively the SEM images of encapsulated matrix before and after oxidant release.
Equation (3) can be represented in the logarithmic form as shown in Equation (4). A plot of logSe against logt, give the slope as n while k is obtained from the intercept. Thus, the time required for every percent release can be obtained from the model.
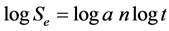 (4)
(4)
For the mass ratio 1:8, a plot of logSe against logt gives the value n = 0.2611 and k = 6.75 × 10−2. Therefore the model can be represented as
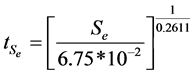 (5)
(5)
4. Conclusion and Recommendation
PMMA was effective in controlling the instant dissolution of KMnO4 in aqueous media. The release rate is very low and depends on the thickness of the PMMA layer on oxidant surface. Results showed that the encapsulation of KMnO4 by PMMA using the modified molten suspension technique controlled the rate of dissolution of KMnO4. The interaction mechanism between PMMA and KMnO4 during encapsulation is not discussed in this paper. The analysis of release data attributed to crevices obtained on the surfaces of PMMA is according to models for other glassy polymers. This study provides insight into the application of PMMA in oxidant release and persistence in long-term leaching organic contamination.