1. Introduction
Lipofuscin-like pigments (LFPs) are considered as the cumulative index of oxidative stress and the hallmark of cellular aging [1] . They are brown-yellow, autofluorescent, electron-dense, polymeric materials formed in many tissues through aging process and also under different pathological conditions. These pigments are found both in cells with mitotic capacity, such as hepatocytes, smooth muscle cells, and a variety of cells in other tissues as the kidney, thymus, pancreas, testis, prostate, uterus, and adrenal gland [2] [3] and in postmitotic cells such as neurons, cardiac muscle and retinal pigment epithelial (RPE) [2] [4] . Although lipofuscin and LFPs composition significantly varies among different types of cells, all these pigments are heterogeneous, high-molecular weight granular material and undegradable, most likely due to the presence of highly oxidized cross-linked aggregates consisting of oxidized protein (30% - 58%) and lipid (19% - 51%) clusters [2] [5] [6] .
More than 100 years have passed since the recognition of these pigments, but their deleterious effects on cell function have been determined in recent years. These studies have shown that free radicals play a key role in the formation of lipofuscin and the relevant damage in tissues [5] . The accumulation of these pigments can be the inevitable consequence of normal oxygen metabolism, which is associated with a surplus formation of reactive oxygen species (ROS) [7] . In addition, the range of exogenous factors such as different kinds of radiation, exposure to heavy metals, drugs and xenobiotics and carbon tetrachloride (CCl4) may induce the lipofuscin and LFPs formation [8] - [11] .
CCl4 is a lipid-soluble toxic agent used commonly to induce oxidative stress in rats [11] . Several studies have indicated that CCl4 produces free radicals with consequent harmful damage to tissues such as the liver, kidneys, heart, lung and brain as well as blood [11] . One mechanism behind these damaging effects is the formation of trichloromethyl (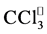 ) and trichloromethylperoxyl (
) and trichloromethylperoxyl (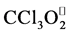 ) radicals and the covalent binding of
) radicals and the covalent binding of 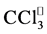 to vital target molecules such as DNA, lipids, proteins and carbohydrates. These free radicals also can initiate lipid peroxidation of poly unsaturated fatty acids (PUFA) of the biological membrane lipids, leading to formation of lipid peroxides. These peroxides are unstable and their decomposition products react with various cellular materials to create fluorescent end-products called lipofuscin and LFPs [2] [10] [12] .
to vital target molecules such as DNA, lipids, proteins and carbohydrates. These free radicals also can initiate lipid peroxidation of poly unsaturated fatty acids (PUFA) of the biological membrane lipids, leading to formation of lipid peroxides. These peroxides are unstable and their decomposition products react with various cellular materials to create fluorescent end-products called lipofuscin and LFPs [2] [10] [12] .
The involvement of oxidative stress in the formation of lipofuscin and LFPs indicates that antioxidants might have beneficial effects on reducing the amount of LF and LFP pigments and the other age-related damages. In that regard, extensive research is ongoing in the world to find natural or synthetic novel compounds to fight the formation of these pigments. The large size, short circulating half-life, antigenisity, cellular permeability, instability and the expense of the antioxidant enzymes have limited their use as therapeutic agents. Therefore, universal attention has been devoted to natural/synthetic small molecules that mimic antioxidant functions of some of the endogenous enzymes as SOD and CAT [13] . Mn-macrocyclic complexes, Mn-salen complexes and Mn- porphyrins are three major classes of these compounds. The Mn-macrocyclic complexes have SOD activity, but not other ROS scavenging properties. The Mn-porphyrins and Mn-salen complexes are synthetic compounds with both SOD and catalase activities [14] [15] . Furthermore, these complexes have the ability of reacting with nitrite ion and lipid peroxides and thus, they have shown efficacy in a wide variety of biological models for ROS-associated diseases [13] . Mn-salens complexes are a special group of synthetic antioxidants with a better cellular penetrability, solubility, and low-molecular weight among enzymatic-like antioxidants. In addition, their catalytic properties make them more potent than traditional small antioxidant compounds. Such properties make Mn-salen complexes not only useful as potential therapeutic agents, but also as valuable tools for studying the role of ROS in many oxidative damages and pathologies associated with ROS [13] [15] . Studies have shown that these compounds could protect injuries due to oxidative damage in animal models of ROS-associated diseases such as Alzheimer’s disease, Parkinson’s disease, inflammatory autoimmune disease (IAD), ischemia, epilepsy, amyotrophic lateral sclerosis (ALS) and stroke [16] [17] . EUK-134 attenuates kainite-induced neuropathology, and significantly decreases DNA-binding activity of two transcription factors, activator protein-1 (AP-1) and NF-κB [16] [17] . Furthermore, EUK-189 has been known as a neuroprotective agent to increase survival, to suppress oxidative pathologies in mice lacking Mn-SOD, to prolong lifespan in the nematode C. elegans, to attenuate age-associated learning impairment and to protect heat-induced liver injury in old rats [14] . The present investigation has evaluated the beneficiary of post-treatment with Mn-salen derivatives on the extent of lipofuscin formation in the CCl4-exposed rats.
2. Materials and Methods
2.1. Chemicals
Reduced glutathione (GSH) and oxidized glutathione (GSSG) were obtained from Fluka (Buchs, Switzerland). Ascorbic acid, trichloroacetic acid (TCA), 2,4-dinitrophenylhydrazine (DNPH), EDTA, glutathione reductase (GR), CCl4, chloroform, methanol and Tris buffer-HCl were obtained from Sigma-Aldrich Chemical Co. Ltd. (England). Bovine serum albumin (BSA), Nitroblue tetrazolium (NBT), 5,5’-dithiobisnitro benzoic acid (DTNB), phenazine methosulfate (PMS), thiobarbituric acid (TBA), nicotinamide adenine dinucleotide reduced (NADH), nicotinamide adenine dinucleotide phosphate reduced (NADPH), hydrogen peroxide (H2O2) and butylated hydroxytoluene (BHT) were obtained from Merck Co. (Germany). 2’,7’-Dichlorofluorescein diacetate (DCFH-DA) was purchased from Molecular Probe (Eugene, OR). All other chemicals used were analytical grade. Mn-salen derivatives were synthesized according to the method of Doctrow et al. [18] (Figure 1) and their relevant precursors were purchased from Merck or Fluka companies.
2.2. Animals, Experimental Design and Preparation of Homogenates
Young male wistar rats, weighting 200 - 220 g, obtained from Tehran University (Tehran, Iran), were housed under light (12 h light/dark cycle) and ambient temperature (22˚C ± 2˚C) with relative humidity of 60% ± 5%. The animals received food and water ad libitum. After the adaptation period, rats were randomly divided into nine groups of six animals each: Group I (normal rats) were administered olive oil intraperitoneally (i.p.) at a dose of 2 ml/kg b.w., for 6 consecutive days; Group II (CCl4 intoxicated) were administered vehicle and CCl4 (50% CCl4 in olive oil, 2 ml/kg b.w., i.p.) on the first and second days, and vehicle (2 ml/kg b.w., i.p.) on the third to sixth days; Groups III-IX (test rats) were administered EUKs 8, 134, 15, 115, 122, 132 and/or vitamin C as a standard antioxidant, respectively (20 mg/kg b.w., orally) and CCl4 in olive oil (2 ml/kg b.w., i.p.) on the first and second days and also the same EUKs and/or vitamin C (20 mg/kg b.w., orally) on the third to sixth days. All experiments were carried out according to the guidelines for the care and use of experimental animals, approved by the state veterinary administration of University of Tehran. At the end and following an overnight fast, the rats were anesthetized by ether and were sacrificed. The liver and brain were rapidly removed and weighted.
![]()
Figure 1. Structures of Mn-salen derivatives (compounds 1-6). Substituents include methoxy (OMe); fluoride (F−) and chloride (X).
A portion of each tissue was fixed in 10% formalin solution for histopathological examinations and the remainder rinsed with a cold PBS solution, blotted on filter paper and then stored at −70˚C. The homogenization was done in 50 mm phosphate buffer (pH 7.4) to give a 10% (w/v) homogenate and centrifuged at 10,000 g, 4˚C, for 10 min. Each supernatant was stored at −70˚C for further biochemical analysis. The protein content was determined based on Lowr’s method using crystalline bovine serum albumin (BSA) as the standard [19] . In addition, blood samples were obtained at the time of sacrifice for subsequent analyses.
2.3. Biochemical Analysis
The serum was obtained after centrifugation of the blood samples at 4˚C, 3000 rpm for 15 min and the aliquots were stored at −20˚C. The serum levels of glucose (GLU), total cholesterol (TC), triglyceride (TG) and albumin were measured by using commercial kits (Pars Azmoon, Tehran, Iran). The activity of alanine aminotransferase (ALT), aspartate aminotransefrase (AST) Alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) in serum were determined using commercial enzymatic kits (Pars Azmoon, Tehran, Iran) as well.
2.4. Assays of Oxidative Biomarkers
ROS levels in each reaction mixture were measured by following the oxidation of 2’,7’-dichlorofluorescein diacetate (DCFH-DA) to the highly fluorescent compound, 2’,7’-dichlorofluorescein (DCF), according to a previously published method with slight modification [20] . Each sample contained 1.85 ml of phosphate buffer (50 mm, pH 7.4) solution, 0.1 ml of tissue homogenate, and 50 µl of DCFH-DA solution (10 µm). The samples were incubated in a bath at 37˚C for 15 min. The ROS concentrations were measured via the formation of DCF using a spectrofluorometer (model Cary Eclipse; Varian Inc., Palo Alto, CA) with the excitation (EX) and emission (EM) wavelengths at 488 and 525 nm, respectively.
Lipid peroxidation was measured by the method of Draper et al. [21] . The method is based on the spectrophotometeric measurement of the purple color generated by the reaction of MDA with thiobarbituric acid (TBA). Briefly, 0.5 ml of tissue homogenate was mixed with 2.5 ml of tricholoroacetic acid (TCA, 10%, w/v) solution. The mixture was then heated in a boiling water bath for 15 min. After cooling to room temperature, the samples were centrifuged at 3000 rpm for 10 min. 2 ml of each sample supernatant was transferred to a test tube contain- ing 1 ml of TBA solution (0.67%, w/v). Then, each tube was placed in a boiling water bath for 15 min. After cooling to room temperature, the absorbance was measured at 532 nm with respect to the blank solution. The concentration of MDA was calculated based on the absorbance coefficient of the TBA-MDA complex (ε = 1.56 × 105 cm−1∙M−1) and it was expressed as nmol/mg protein.
The PCO levels in the homogenates were measured using the method of Reznick et al. (1994) [22] . Briefly, 2 ml of 10 mm DNPH in 2.5 M HCl was added to 1 ml of each tissue homogenate. Samples were incubated for 1 hour at room temperature and vortexed every 10 - 15 min. Then, 2.5 ml of cold trichloroacetic acid (TCA) (20%, w/v) was added to each reaction mixture and centrifuged at 3000 g for 10 min. The protein pellet was washed three times with 2 ml of ethanol/ethyl acetate (1:1, v/v) and dissolved in 1 ml of guanidine hydrochloride (6 M, pH 2.3). The product was incubated for 20 min at 37˚C and vortexed. The carbonyl content was calculated, based on the molar extinction coefficient of DNPH (ε = 2.2 × 104 cm−1∙M−1). The data were expressed in terms of nmole/mg protein.
2.5. Assay of LFPs
LFPs were extracted based on the technique described by Fletcher et al. (1973) with slight modification [23] . To 500 µl of each frozen homogenate, 2 ml of chloroform-methanol mixture (2:1, v/v) was added and the suspension was vortexed vigorously for a few minutes. Then, 1 ml of distilled water was added to achieve phase separation, mixed and the samples were centrifuged at 3000 rpm for 10 min. After centrifugation, the lower chloroform phase was separated from the aqueous phase and both phases used for the assays. The fluorescence intensity was measured at EX/EM of 363/430 nm and 377/430 nm for organic and aqueous phases, respectively, using a Varian spectrofluorometer, model Cary Eclipse.
2.6. Assays of Antioxidant Enzyms
CAT activity was determined according to the method of Aebi by spectrophotometric analysis of the decomposition rate of hydrogen peroxide [24] . An aliquot (5 µl) of each tissue supernatant was added to a cuvette containing 1.995 ml of 50 mm phosphate buffer (pH 7.0). The reaction was started by addition of 1.0 ml of freshly prepared 30 mm H2O2. The rate of decomposition of H2O2 was measured after 15, 30, 45 and 60 min spectrophotometrically at 240 nm. The activity of CAT was expressed as ×10−1 k/mg protein, where k represents the rate constant of the first order reaction of CAT.
SOD activity was measured based on the extent inhibition of amino blue tetrazolium formazan formation in the mixture of nicotinamide adenine dinucleotide, phenazine methosulphate and nitroblue tetrazolium (NADH- PMS-NBT), according to method of Kakkar [25] . Assay mixture contained 0.1 ml of the supernatant, 1.2 ml of sodium pyrophosphate buffer (pH 8.3; 0.052 M), 0.1 ml of phenazine methosulphate (186 µm), 0.3 ml of nitroblue tetrazolium (300 µm) and 0.2 ml of NADH (750 µm). Reaction was started by addition of NADH. After incubation at 30˚C for 1.5 min, the reaction was stopped by addition of 0.1 ml of glacial acetic acid. Reaction mixture was stirred vigorously with 4.0 ml of n-butanol. Colour intensity of the chromogen in the butanol was measured spectrophotometrically at 560 nm. One unit of enzyme activity was defined as that amount of enzyme which caused 50% inhibition of NBT reduction/mg protein.
The GR level in each homogenate was assayed by a reaction mixture containing 0.99 ml of 100 mm potassium phosphate buffer (pH 7.0), 10 mm MgCl2, 10 mm oxidized GSSG and 1 mm NADPH. From each homogenate, 10 µl was added to trigger the NADPH conversion reaction. Changes in absorbance were monitored at 340 nm for 5 min at 25˚C. The specific enzyme activity of GR was expressed as nmol NADPH oxidized to NADP+/min/mg protein with 6.22 × 106 (cm−1∙M−1) as the molar extinction coefficient of NADPH [26] .
The GPx level in each homogenate was assayed in a 1 ml cuvette containing 890 µl of 100 mm potassium phosphate buffer (pH 7.0), 100 µm EDTA, 100 µm NaN3, 200 µm NADPH, 1 U/ml GR and 1 mm GSH. From each homogenate, 10 µl was added to make a total volume of 900 µl. The reaction was initiated by the addition of 100 µl of 2.5 mm H2O2, and the conversion of NADPH to NADP+ was monitored with a spectrophotometer at 340 nm for 3 min. GPx activity was expressed as nmoles of NADPH oxidized to NADP+/min/mg protein, using a molar extinction coefficient of 6.22 × 106 (cm−1∙M−1) for NADPH [27] .
2.7. Assay of Non-Enzymatic Antioxidants
GSH was estimated by the method of Jollow et al. [26] . An aliquot of 0.5 ml of each tissue homogenate was first precipitated with 1 ml of sulphosalicylic acid (4% w/v) at 4˚C. The precipitate was removed by centrifugation at 3000 rpm, 4˚C and for 10 min. The filtered sample (1 ml) was mixed with 0.1 ml DTNB (4 mg/ml) and 0.9 ml phosphate buffer (0.1 M, pH 7.4). The yellow colour developed was measured at 412 nm. Reduced glutathione was expressed as µg/mg of protein.
2.8. Histopathological Examinations
Prevalent techniques of paraffin-wax sectioning and haematoxylin-eosin staining were used for histological studies [28] . Slices of fresh liver and brain tissues were cut and kept in 10% formalin solution. Following fixation, paraffin-embedded blocks were prepared and stained with hematoxylin-eosin and Masson-trichrome. The evaluations were achieved by a skillful pathologist and examined by a bright-field microscope at a magnification of 400×.
2.9. Statistical Analyses
All data were expressed as mean ± S.D., statistical significance of the differences between groups was calculated by Student’s t-test, and p < 0.05 was considered significant.
3. Results
In this study, protective effects of Mn-salen derivatives against CCl4-induced damages were evaluated in male rats by spacious evaluation of liver and brain functions and oxidative biomarkers. The doses of CCl4 and/or EUKs were chosen on the basis of preliminary studies (data not shown) and the experiments were carried out at a CCl4 concentration of 2 ml/kg b.w./day for 2 days and post-treatment of EUKs at a concentration of 20 mg/kg b.w./day for four days.
3.1. Serum Biochemical Evaluations
Biochemical analyses of the sera were performed to study possible changes in enzymatic biomarkers, GLU, lipid profile and albumin of the control and the test rats. In this study, the administration of CCl4 to rats caused 145%, 117%, 47% and 53% increase in sera enzymatic biomarkers including AST, ALT, ALP and GGT as well as 36%, 65% and 83% augmentation in sera GLU, TC and TG, respectively. However, post-treatment of rats with EUKs (compounds 1-6) and vitamin C at a dose of 20 mg/kg b.w./day for four days attenuated the CCl4-induced deleterious effects in blood and reduced the elevated AST activity by 145%, 94%, 131%, 9%, 125%, 116%, 90%, ALT activity by 119%, 79%, 89%, 42%, 81%, 72%, 44%, ALP activity by 47%, 20%, 16%, 4%, 15%, 11%, 12% and GGT activity by 36%, 52%, 51%, 35%, 42%, 34% and 33%, respectively relative to rats treated solely with CCl4 (Figure 2). Also, EUKs (compounds 1-6) post treatments decreased the sera levels of GLU by about 17%, 6%, 8%, 15%, 30%, 32%, 29%, TC by about 24%, 48%, 53%, 21%, 37%, 41%, 33% and TG by about 48%, 69%, 53%, 47%, 60%, 70% and 45%, respectively relative to CCl4-exposed rats. Whereas, the compounds (1-6) caused a slight increase in the sera levels of albumin by 8%, 10%, 9%, 7%, 11%, 11% and 9%, respectively relative to CCl4-exposed rats (Table 1). Based on these results, it might be concluded that Mn-salen derivatives have protective effects against CCl4-induced destructive damage. In that respect, EUKs 8, 15, 122 and 134 showed higher activity than that of EUKs 115, 132 and vitamin C toward the hepatic enzymes in the sera of the treated rats. EUKs 134, 15, 122 and 132 with ortho/para substitutions on salen rings were more effective than the other compounds in decreasing lipid factors. EUKs 122 and 132 were more effective in reduction of glucose level as well.
![]()
Figure 2. Effect of Mn-salen derivatives (compounds 1-6) on the serum activities of AST, ALT, ALP and GGT in rats. Rats were pre-treated first with 2 ml/kg b.w./day of CCl4 for 2 days, then they were treated with Mn-salen derivatives (20 mg/kg b.w.) for 4 days. Each salen stock solution was prepared at 10 mg/ kg b.w./day, and the experiments were performed orally with 2 ml of this solution. The activities of AST, ALT and ALP were expressed in IU/L. The activity of GGT expressed in IU/L × 10−1. Each value represents the mean ± SD (n = 3) of six rats per group. #Significantly different from normal rats (p < 0.05). *Significantly different from CCl4-exposed rats (p < 0.05).
![]()
Table 1.Effect of Mn-salen derivatives (compounds 1-6) on the serum GLU, TC, TG and ALB levels of the rates.
Rats treated with Mn-salen derivatives (20 mg/kg b.w) for 4 days, after treatment of 2 ml/kg b.w/day CCl4 for 2 days. The levels of GLU, TC, TG and ALB expressed in mg/dl. Each value represents the mean ± SD (n = 3) of six rats per group. #Significantly different from normal rats (p < 0.05). *Significantly different from CCl4-exposed rats (p < 0.05).
3.2. Inhibitory Effects of Mn-Salen Derivatives on the ROS Level
Physiological imbalance between oxidants and antioxidants leads to the so-called oxidative stress condition and an increase in the intracellular levels of ROS. Our data indicated that post-treatments with EUKs (compounds 1- 6) reduced CCl4-induced harmful effects in both the liver and brain tissues. As indicated in Figure 3, CCl4-ex- posed rats showed 43% and 38% increase in the levels of ROS in liver and brain, respectively relative to normal rats. However, Post-treatment of rats with EUKs (compounds 1-6) and vitamin C at a dose of 20 mg/kg b.w./day for four days suppressed the levels of ROS, by 49%, 41%, 24%, 39%, 38%, 25% and 44% in liver and by 18%, 0%, 21%, 5%, 5%, 3% and 1% in brain, respectively relative to rats treated solely with CCl4 (Figure 3). Among EUKs, EUKs 8, 134, 122, 115 and vitamin C in liver and EUKs 8 and 15 in brain had more efficiency than the other compounds. EUKs 134, 115, 122, 132 and vitamin C had almost no effects on the ROS levels in the brains.
3.3. Inhibitory Effects of Mn-Salen Derivatives against Protein Oxidation and Lipid Peroxidation
Lipid peroxidation is initiated by the interaction of ROS with polyunsaturated fatty acids of membrane lipids, leading to a number of degradation products. MDA is one of the products of lipid peroxidation which is referred to as an index of lipid peroxidation and as a marker of oxidative stress. As summarized in Table 2, CCl4 administration caused 110% and 50% increase in the levels of MDA, respectively in the CCl4-exposed-rats’ liver and brain relative to normal rats. However, Post-treatment of rats with EUKs (compounds 1-6) and vitamin C at a dose of 20 mg/kg b.w./day for four days inhibited MDA formation by 52%, 67%, 52%, 10%, 5%, 0%, 71% in liver and by 53%, 40%, 43%, 20%, 13%, 0% and 50% in brain, respectively relative to CCl4-exposed rats.
In addition, the protein oxidation was evaluated in terms of the PCO content as an index for oxidative damages of proteins. As shown in Table 2, the levels of PCO increased in CCl4-exposed-rats’ liver and brain relative to normal rats by 37% and 56%, respectively. The extent of PCO formation was quenched by these derivatives (compounds 1-6) and vitamin C by 29%, 40%, 21%, 1%, 32%, 20%, 25% in liver and by 14%, 51%, 14%, 1%, 44%, 34% and 52% in brain, respectively compared to CCl4-exposed rats.
Collectively, the results indicated that among EUKs, EUKs 134, 122 and 8 in liver and EUKs 134, 122 and 132 in brain showed the highest activity with respect to the reduction of protein oxidation relative to others. In that respect, EUK-134 had the highest function and EUK-115 had the lowest efficacy in suppression of oxidative intensity in both liver and brain tissues. Furthermore, EUKs 8, 15 and 134 exhibited the most ability in the prevention of lipid peroxidation relative to CCl4-exposed rats. Vitamin C showed significant role in reduction of protein oxidation and lipid peroxidation in both tissues as well.
![]()
Figure 3. Inhibitory effects of Mn-salen derivatives (compounds 1-6) on the extent of ROS formation level in rats׳ liver and brain tissues. Rats were pre-treated first with 2 ml/kg b.w./day of CCl4 for 2 days, then they were treated with Mn-salen derivatives (20 mg/kg b.w.) for 4 days. Each salen stock solution was prepared at 10 mg/ kg b.w./day, and the experiments were performed orally with 2 ml of this solution. The levels of ROS were measured with DCFH-DA. Data were expressed as percentages of values of normal tissues. Each value represents the mean ± SD (n = 3) of six rats per group. #Significantly different from normal rats (p < 0.05). *Significantly different from CCl4-exposed rats (p < 0.05).
![]()
Table 2. Inhibitory effect of Mn-salen derivatives (compounds 1-6) on the CCl4-induced protein oxidation (PCO) and lipid peroxidation (MAD) in rats liver and brain tissues.
Rats treated with Mn-salen derivatives (20 mg/kg b.w.) for 4 days, after treatment of 2 ml/kg b.w./day CCl4 for 2 days. Lipid peroxidation level expressed as nmol of MDA/ mg protein. Protein oxidation level expressed as nmol of carbonyl/ mg protein. Each value represents the mean ± SD (n = 3) of six rats per group. #Significantly different from normal rats (p < 0.05). *Significantly different from CCl4-exposed rats (p < 0.05).
3.4. Inhibitory Effects of Mn-Salen Derivatives on Lipofuscin-Like Pigment Formation
Lipofuscin-like pigments, as an index of oxidative stress and a hallmark for aging, are mainly made of highly oxidized proteins and lipids. The alterations in the level of these pigments were studied based on their autofluorescent properties. According to Figure 4, exposure of rats to 2 ml/kg b.w./day CCl4 for 2 days caused 50 and 54% increase in the level of (organic phase) lipofuscin-like pigments in liver and brain, respectively relative to normal rats. Post-treatment of the rats with each Mn-salen derivative (compounds 1-6) and vitamin C at a dose of 20 mg/kg b.w./day for four days diminished the formation of these pigments by 13%, 4%, 8%, 28%, 24%, 24%, 18% in liver and by 40%, 28%, 31%, 11%, 19%, 17% and 36% in brain, respectively relative to CCl4-ex- posed rats (Figure 4). Also, the levels of LFPs formation increased in CCl4-exposed-rats’ liver and brain relative to normal rats by 34 and 32%, respectively in the aqueous phase of the extract (Figure 4). However, treatments with the derivatives (compounds 1-6) caused reduction by 53%, 20%, 28%, 50%, 24%, 26 % and 22% in liver and by 20%, 39%, 41%, 1%, 27%, 22% and 2% in brain, respectively compared to CCl4-exposed rats (Figure 4). The results indicated that LFPs accumulations in the organic phase were much more than those of aqueous phase under influence of CCl4. In addition, the results clearly showed that EUKs 8, 134, 15 and vitamin C significantly decreased LFPs content of the organic phase of the brain extracts relative to CCl4-exposed rats, whereas significant relevant changes were not observed in the liver. However, significant changes in the lipofuscin content of the aqueous phase of both the liver and brain extracts were observed relative to CCl4 exposed rats.
3.5. Effects of Mn-Salen Derivatives on Endogenous Antioxidant Enzymes
The alteration of endogenous antioxidant status was determined with the measurement of the activities of CAT, SOD, GR and GPx in liver and brain tissues. As presented in Figure 5, the activities of CAT, SOD, GR and GPx were lowered in rats’ livers by 43%, 39%, 25% and 39% after treatment with CCl4 (2 ml/kg b.w./day, for 2 days). However, Post-treatment of the rats with EUKs (compounds 1-6) and vitamin C at a dose of 20 mg/kg b.w./day for four days augmented the hepatic CAT activity by 60%, 77%, 41%, 38%, 57%, 56%, 65%, SOD activity by 57%, 65%, 31%, 35%, 55%, 52%, 60%, GR activity by 7%, 22%, 10%, 13%, 16%, 22%, 23% and GPx activity by 13%, 35%, 25%, 27%, 31%, 26% and 33%, respectively relative to CCl4-exposed rats(Figure 5).
Furthermore, as indicated in Figure 5, the activities of CAT, SOD, GR and GPx decreased in rats’ brain by 43%, 39%, 35% and 39%, respectively, after CCl4 administration. Following treatments with EUKs (compounds 1-6) and vitamin C, the brain CAT activity increased by 20%, 7%, 27%, 23%, 23%, 23%, 0% , SOD activity by 19%, 4%, 24%, 27%, 19%, 19%, 4%, GR activity by 19%, 23%, 24%, 47%, 42%, 41%, 11% and GPx activity by 23%, 27%, 27%, 51%, 41%, 40% and 20%, respectively relative to CCl4-exposed rats (Figure 5). Collectively, the results confirmed high antioxidant activity of these compounds in in vivo experiments.
![]()
Figure 4. Inhibitory effects of Mn-salen derivatives (compounds 1-6) on the CCl4-induced accumulation of lipofuscin-like pigments in rat’ liver and brain tissues. Rats were pre-treated first with 2 ml/kg b.w./day of CCl4 for 2 days, then they were treated with Mn-salen derivatives (20 mg/kg b.w.) for 4 days. Each salen stock solution was prepared at 10 mg/kg b.w./day, and the experiments were performed orally with 2 ml of this solution. The extent of lipofuscin in each hemogenate was evaluated using a Varian spectrofluometer, model Cary Eclipse, set at an excitation wavelength of 363 nm and an emission wavelength of 430 nm in or- ganic phase and an excitation wavelength of 377 nm and an emission wavelength of 430 nm in aqueous phase (inset). Each value represents the mean ± SD (n = 3) of six rats per group. #Significantly different from normal rats (p < 0.05). *Significantly different from CCl4-exposed rats (p < 0.05).
![]()
Figure 5. Effect of Mn-salen derivatives (compounds 1-6) on the activities of CAT, SOD, GR and GPx in rats’ liver and brain (inset) tissues. Rats were pre-treated first with 2 ml/kg b.w./day of CCl4 for 2 days, then they were treated with Mn-salen derivatives (20 mg/kg b.w.) for 4 days. Each salen stock solution was prepared at 10 mg/kg b.w./day, and the experiments were performed orally with 2 ml of this solution. The activity of liver CAT was expressed in k × 10−1 /mg protein. The activity of brain CAT was expressed in k × 5 × 10−1 /mg protein. The activity of SOD was expressed in U/mg protein. The activities of GR and GPx were expressed in nmol NADPH/mg protein. Each value represents the mean ± SD (n = 3) of six rats per group. #Significantly different from normal rats (p < 0.05). *Significantly different from CCl4-exposed rats (p < 0.05).
3.6. Effects of Mn-Salen Derivatives on CCl4-Induced GSH Depletion
Glutathione, as a non-enzymatic antioxidant, plays an important role in endogenous antioxidant defense mechanisms. As shown in Figure 6, exposure of the rats to 2 ml/kg b.w./day CCl4 for 2 days caused 28 and 34% increase in the GSH level, respectively in the liver and brain relative to normal rats. However, Post-treatment of the rats with each Mn-salen derivative (compounds 1-6) and vitamin C at a dose of 20 mg/kg b.w./day for four days inhibited CCl4-induced GSH depletion by 44%, 26%, 45%, 32%, 31%, 22%, 20% in liver and 17%, 13%, 28%, 37%, 39%, 49% and 5% in brain of rats relative to CCl4-exposed rats (Figure 6). Regarding the inhibition of GSH pool size depletion, EUKs 8 and 15 in liver and EUKs 132, 122, 115 and 15 in brain acted better than the other compounds.
3.7. Histopathological Evaluations
The comparison of the histo-architecture of liver and brain of CCl4-treated relative to normal rats indicated the occurrence of steatosis, ballooning degeneration and inflammation in the liver and the vacuolation around some of cortical neurons and shrunken nuclei in the cerebral cortex cells of the brain tissue (Figure 7(I-A) and Figure 7(I-B), Figure 7(II-A) and Figure 7(II-B)). Based on Figure 7(I-A), the liver samples of the normal rats preserved lobulovascular architecture, individual hepatocytes showed mild ballooning degeneration, the portal tracts were devoid of significant inflammation or necrosis and steatosis. In Figure 7(II-A), the brain samples of the normal rats did not show any vacuolization and shrunken nuclei. Post-treatment with each of the EUKs (compounds 1-6) and vitamin C showed significant effects on the extent of cellular damage caused by CCl4. Each sample showed nearly normal liver architecture with moderate ballooning degeneration and mild lobular inflammation (Figure 7(I-C) to Figure 7(I-I)). However, minimal changes were observed in the cerebral cortex cells of the EUKs-treated rats relative to normal ones. The vacuolization around neurons and shrunken nuclei were not observed among the cerebral cortex cells of the brain tissues. Based on these results, all compounds had either significant or mild protective effects on brain tissue against CCl4 induced damage (Figure 7(II-C) to Figure 7(II-I)).
4. Discussion
This study has focused on the ameliorative effect of Mn-salen derivatives against CCl4-induced oxidative damage in liver and brain of rats. Various studies have demonstrated that CCl4 is an effective reagent for induction of oxidative stress in animal models [12] [29] .
CCl4 is activated by cytochrome P450 2E, 2B1 or 2B2 and possibly CYP 3A [30] , to induce lipid peroxidation through trichloromethyl radical (?CCl3) and trichloromethyl peroxy radicals (?CCl3O2) formation, with subsequent covalent binding of ?CCl3 to membrane proteins and lipids [29] , leading to their eventual cross-linking [31] [32] and the formation of lipofuscin-like pigments (LFPs). This finding underlies an attractive correlation between free radical damage and aging process. The free radical theory of aging proposed by Harman in 1956 is now one of the leading theories explaining the biochemical basis of lipofuscin and LFPs formation. Thus, exogenous antioxidant supplementation under abnormal oxidant state of the biological system seems to be a logical approach to figth abnormal levels of free radicals. In that regard, we evaluated the role of six Mn-salen
![]()
Figure 6. Effect of Mn-salen derivatives (compounds 1-6) on the reduced glutathione (GSH) in rats’ liver and brain tissues. Rats were pre-treated first with 2 ml/kg b.w./day of CCl4 for 2 days, then they were treated with Mn-salen derivatives (20 mg/kg b.w.) for 4 days. Each salen stock solution was prepared at 10 mg/kg b.w./day, and the experiments were performed orally with 2 ml of this solution. Each value represents the mean ± SD (n = 3) of six rats per group. #Significantly different from normal rats (p < 0.05). *Significantly different from CCl4-exposed rats (p < 0.05).
![]()
Figure 7. Histological evaluation of rats’ livers (7-I) and brain (7-II). Photographs of tissues after Hematoxylin-eosin (A-I). (A) Normal tissues, (B) CCl4-exposed rats’ tissues, (C) CCl4 + EUK-8, (D) CCl4 + EUK-134, (E) CCl4 + EUK-15, (F) CCl4 + EUK-115, (G) CCl4 + EUK-122, (H) CCl4 + EUK-132, (I) CCl4 + vitamin C.
derivatives on CCl4-induced oxidative damage in rats’ livers and brains.
According to the literature data, radicals formed through metabolic conversion of CCl4, initiate the peroxidation of membrane poly-unsaturated fatty acids (PUFA) [11] [30] [33] with ultimate production of MDA and 4-HNE [3] [33] . These aldehydes are believed to be the main indicator of age pigment formation. Also, oxidative damage to amino acid residues of peptides and proteins results in the production of PCO by-products. Among the various amino acids, tyrosine, tryptophan, phenylalanine, histidine, cysteine, methionine, and lysine are the most vulnerable to oxidative modification [3] [22] .
Mn-salen complexes, consisting of manganese ion and a salen ligand, are synthetic catalytic antioxidants with the enzymatic-like function. Manganese ion in the complex is able to participate in various recycling redox reactions, reacts with reactive oxygen species such as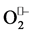 , H2O2 and lipid peroxides. The type and situation of substituents on the salen ring can modulate the electron transfer criteria of the central metal ion. Accordingly, Mn-salen derivatives (compounds 1-6) could prevent the Fenton reaction by the capture of free radicals and consequently, decrease protein and lipid peroxidation (PCO and active aldehydes production) and their cross- linking together and eventually attenuate the formation of LFPs accumulations. These compounds were able to cross the blood-brain barrier and among EUKs, EUKs 8, 15 (with a para OMe) and 134 (with an ortho OMe) showed more efficiency in the reduction of brain LFPs accumulation. These results were parallel to our in vitro findings that revealed the majority of Mn-salen derivatives, especially EUKs 15, 123 and 189, quenched ROS, MDA, PCO and LFPs formation according to their radical scavenging activity [34] , whereas the levels of LFP in the livers of EUKs-treated rats were not significant relative to CCl4 group, possibly because of metabolic activity of liver on EUKs. Our observations is consistent with the previously published results showing that the elevated levels of ROS, MDA and PCO were suppressed in tissues such as liver, kidney, heart and brain of CCl4- treated rats and cells such as SK-N-MC following treatments with a variety of natural and synthetic antioxidants [11] [12] [30] [33] [35] [36] .
, H2O2 and lipid peroxides. The type and situation of substituents on the salen ring can modulate the electron transfer criteria of the central metal ion. Accordingly, Mn-salen derivatives (compounds 1-6) could prevent the Fenton reaction by the capture of free radicals and consequently, decrease protein and lipid peroxidation (PCO and active aldehydes production) and their cross- linking together and eventually attenuate the formation of LFPs accumulations. These compounds were able to cross the blood-brain barrier and among EUKs, EUKs 8, 15 (with a para OMe) and 134 (with an ortho OMe) showed more efficiency in the reduction of brain LFPs accumulation. These results were parallel to our in vitro findings that revealed the majority of Mn-salen derivatives, especially EUKs 15, 123 and 189, quenched ROS, MDA, PCO and LFPs formation according to their radical scavenging activity [34] , whereas the levels of LFP in the livers of EUKs-treated rats were not significant relative to CCl4 group, possibly because of metabolic activity of liver on EUKs. Our observations is consistent with the previously published results showing that the elevated levels of ROS, MDA and PCO were suppressed in tissues such as liver, kidney, heart and brain of CCl4- treated rats and cells such as SK-N-MC following treatments with a variety of natural and synthetic antioxidants [11] [12] [30] [33] [35] [36] .
On the other hand, biological systems are supported with various defense mechanisms against environmental and internal stresses [30] . Among them, enzymatic antioxidants and GSH plays a crucial role in the maintenance of cellular oxidative balance in these systems. Our results showed that CCl4 significantly decreased the CAT, SOD, GR and GPx activities and caused depletion of GSH in liver and brain of CCl4-exposed rats relative to normal rats, implying vulnerability of cells to oxidative attack and alterations of redox status probably due to generation of toxic reactive metabolites, reactive oxygen species and products of lipid peroxidation. Independant studies have shown that 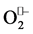 and •OH radicals are able to inhibit the activity of CAT, SOD and Gpx enzymes and even lowering their biosyntheses [30] [37] . Some other studies have revealed that the products of lipid peroxidation have the ability to react with amino acid residues of antioxidant enzymes, leading to their inactivation [34] . Similarly, dialdehydes, products of lipid peroxidation with the ability to react with the thiol groups of proteins, can cause GSH oxidation and hence increase in GSSG:GSH ratio [9] [12] [30] . In current study, post-treatment of rats with the Mn-salen derivatives (compounds 1-6) strengthened the defense system and prevented depletion of GSH probably via either ROS scavenging and/or the stimulation of gene expression of antioxidant enzymes [30] [38] . Present results are complementary to the observations reported earlier about the ameliorative effects of some natural and synthetic antioxidants on the activity of CAT, SOD and GPX as well as the level of GSH in animal and cell culture models [11] [12] [14] [30] [39] .
and •OH radicals are able to inhibit the activity of CAT, SOD and Gpx enzymes and even lowering their biosyntheses [30] [37] . Some other studies have revealed that the products of lipid peroxidation have the ability to react with amino acid residues of antioxidant enzymes, leading to their inactivation [34] . Similarly, dialdehydes, products of lipid peroxidation with the ability to react with the thiol groups of proteins, can cause GSH oxidation and hence increase in GSSG:GSH ratio [9] [12] [30] . In current study, post-treatment of rats with the Mn-salen derivatives (compounds 1-6) strengthened the defense system and prevented depletion of GSH probably via either ROS scavenging and/or the stimulation of gene expression of antioxidant enzymes [30] [38] . Present results are complementary to the observations reported earlier about the ameliorative effects of some natural and synthetic antioxidants on the activity of CAT, SOD and GPX as well as the level of GSH in animal and cell culture models [11] [12] [14] [30] [39] .
Moreover, our results revealed an increase in the activities of the serum hepatic enzymes such as AST, ALT, ALP and GGT upon exposure to CCl4 compared to normal rats, reflecting the CCl4-induced substantial damage of tissues, mainly the liver cells [40] . Treatment with Mn-salen derivatives (compounds 1-6) decreased the activity of these enzymes in the serum, suggesting the ameliorative action of Mn-salen derivatives against the deleterious effects of CCl4 on liver. In addition to enzymatic parameters, administration of CCl4 to rats resulted in elevation of cholesterol and triglycerides in serum. Studies have shown that lipid peroxides gets involved in the oxidation of LDL-C and its receptor (apoprotein B100). This might account for high level of LDL-C. Our results indicated that post-treatment of CCl4-exposed rats with Mn-salens derivatives caused a decline in serum cholesterol and triglyceride levels, indicating their possible inhibition because of reducing their absorption in the gut and/or increasing their excretion from feces and showing the properties of hypocholesterolemic and hypotriglyceridemic Mn-salen derivatives. These results are associated with decreased levels of MDA, as an index of lipid peroxidation. Furthermore, our results showed that serum glucose concentration in CCl4-treated rats had significantly increased compared to the normal group. The higher than normal level of glucose is believed to initiate protein glycations through Maillard reaction leading to production of AGEs products [3] . The high level of protein glycations under hyperglycemic condition might also account for inactivation and lower levels of SOD and CAT activities [41] . In addition, higher than normal level of sorbitol under hyperglycemia condition would lead to higher level of NADP+. To restore the normal level of NADPH, GSH is consumed in a reaction catalyzed by GR, leading to lower than normal level of GSH [42] . Our results indicated that the salen derivatives were effective in GSH level restoration, attenuation of protein modification (PCO) and reduction of blood glucose level in rats, parallel to the relevant literature data [12] [29] [34] [39] [40] [43] .
Our histological results, in agreement with previous studies [30] , demonstrated that CCl4 cause destructive effects on different organs such as the liver and brain. These results revealed that Mn-salen derivatives diminished CCl4-induced abnormalities without adverse effects on rats’ brain. These compounds could overcome the effects of vacuolation around neurons and shrunken nuclei in the cerebral cortex cells of the brain tissue of CCl4-exposed rats. The results also indicated that these compounds attenuated hepatocellular damage, hepatocyte necrosis and ballooning degeneration in the liver of CCl4-exposed rats.
Overall, our data indicated that Mn-salen derivatives have ameliorative action on CCl4-induced destructive effects and lipofuscin-like pigment formation in rats’ liver and brain. The substituents on the salen ring could either enhance or weaken the activity of these compounds probably due to modulation of electron density on the ring through a combination of resonance and inductive effects. The electron donating groups such as OMe usually act through resonance effect and show better action at the para and ortho positions than that at metha position [18] , whereas, halogen groups have dual inductive electron withdrawing effect and resonance electron donating activity [44] . Fluorine is the smallest halogen with the highest electronegativity. Its high electronegativity causes s electrons to be withdrawn away from the ring. Its small size increases the distance between the energetic levels of fluoride HOMO and salen ring LUMO. Thus, the inductive effect of fluoride substituents predominate the resonance effect and thus, the antioxidative activities of the Mn-salen complexes with fluoride substituent is more than OMe group.
Our study demonstrates that CCl4 not only induces tissue damage but also damages enzymatic structure and function, which is amenable to attenuation by Mn-salen derivatives. The results of the biochemical and histological treatments indicate that Mn-salen derivatives (compounds 1-6) permeate through biological membranes and protect liver and brain tissues and blood against CCl4-induced structural damage and functional disorders. The protective effect of Mn-salen derivatives may be due to both their free radical scavenging properties and the indirect effects on the defense systems. As a result, they may be applicable in inhibiting LFPs accumulation and the therapy of age-related diseases. In that respect, EUK-134 (with an ortho OMe) group in liver and EUKs 8 and 15 (with a para OMe) group in brain had much higher ameliorative effect on oxidative biomarkers and enzymatic activities than the other compounds. EUKs 8, 15 and 134 showed the most efficiency in the reduction of brain LFPs accumulation in the organic phase, whereas no significant change in the level of liver LFPs was observed by EUKs. Our data provided the primary background required for pharmaceutical candidacy pending further intensive evaluation of biological and pharmacological evaluation of the compounds.
Acknowledgements
The authors appreciate the financial support of this investigation by the Research Council of University of Tehran.
NOTES
*Corresponding author.