Preparation and Microwave Absorbing Characteristics of Multi-Walled Carbon Nanotube/Chiral-Polyaniline Composites ()
1. Introduction
In recent years, electromagnetic interference (EMI) shielding problems are increasing in electronic and military communication owing to sensitive electronic devices and densely packed systems [1] . A variety of research works have been carried out to investigate the dielectric property, EMI shielding and microwave-absorption [2] -[9] for protecting the workspace, environment and sensitive circuits against microwave radiation emitting from computers and telecommunication equipments [10] . The absorption and the interference shielding of electromagnetic wave have been very important issues for commercial and military purposes. And with the development of radar, the stealth technique becomes the most typical application of electromagnetic wave absorption technology. By reducing the detectability of aircrafts or warships, of which the radar cross-section (RCS) is a measure, they could evade radar detection, which affects not only the mission success rate but also survival of them in the hostile territory.
Microwave absorbers are able to absorb the incident radiation in synchronized frequencies. There are many kinds of electromagnetic absorbers. Compared to conventional metal-based microwave absorbers, conductive polymer composites have gained popularity recently due to their light weight, flexibility, versatility, resistance to corrosion, and low-cost industrial processing [11] -[14] . Generally, electromagnetic wave absorption characteristic of a material depends on many factors, including its dielectric properties, magnetic properties, thickness, frequency range, aspect ratio and the filler’s intrinsic conductivity [1] [11] [15] .
Nano-materials have received increasingly more attention for their potential applications as practical structural and functional materials. Since the discovery in 1991 [16] , carbon nanotubes (CNTs) have attracted more and more interest for their distinguished properties and promising future applications. The unique mechanical properties of CNTs such as their high conductivity, small diameter, the enormous aspect ratio, and super mechanical strength and stiffness make them a potential structural element for creating conductive composites for highperformance EMI shielding materials at low filling concentration [17] . Further potential advantages, such as their electrical and thermal conductivity together with a low density [18] -[23] , make CNTs as ultimate fillers for polymers. MWCNTs exhibit excellent mechanical, thermal, and electrical properties, etc. [24] . Recently, many researchers studied the MWCNTs composites containing different polymer matrix, such as polystyrene (PS) [10] , epoxy [4] , poly (methylmethacrylate) (PMMA) [25] , PANI [10], polypyrrole (PPY) [10] , PU [4] [26] [27] , poly (vinyl alcohol) [28] , etc., and also explored their potential applications.
PANI is one of the most important conducting polymers [29] [30] because of its low cost of its monomer, its easy preparation, its higher conductivity, its unique electrical, optical, and optoelectrical properties and other relevant properties in such application fields as chemoor biosensors, electromagnetic shielding, anticorrosion, electrode materials for secondary batteries, electrochromic devices, and membrane separations [31] [32] . Furthermore, PANI is unique because of its reverse acid-base doping/dedoping behavior [33] [34] . Chiral polymers discriminate between left (L) and right (R) circular polarized light in their optoelectronic properties [33] . Nowadays, chiral polymers are discussed for several applications. There is a special interest in chiral functional polymers [34] . With regard to the use of chiral materials as electromagnetic wave absorbers, Varadan et al. first reported that conductive chiral polymers showed excellent absorption properties of electromagnetic waves [35] .
In this paper, we prepared MWCNTs/chiral-PANI composites using the conventional chemical oxidative polymerization of aniline(ANI) in the presence of (1S)-(+)-10- or (1R)-(−)-10-camphorsulfonic acid. An EMI shielding efficiency up to −14.93 dB at the frequency of 6.7 GHz was obtained and EMI shielding mechanism of MWCNTs/chiral-PANI composites were studied. The contribution of PANI’s chirality and its doping ratio to EMI shielding efficiency were also investigated.
2. Experimental
2.1. Materials
The MWCNTs were purchased from Chengdu Organic Chemicals Co. Ltd., Chinese Academy of Sciences (COCC), they were synthesized by a chemical vapor deposition method with a carbon mass fraction of about 95% and were 0 - 8 nm in diameter and 10 - 30 μm in length. ANI and concentrated ammonium hydroxide solution were purchased from Fu Chen Chemical Technology Company Ltd., Shanghai, China. ANI was distilled under vacuum prior to usage. Concentrated ammonium hydroxide was diluted with deionized water for the dedoping experiments. (1S)-(+)-10-camphorsulfonic acid (D-CSA, 99% purity), and (1R)-(-)-10-camphorsulfonic acid (L-CSA, 99%) were purchased from Dibo Chemical Technology Company Ltd., Shanghai, China.
2.2. Synthesis of MWCNTs/Chiral-PANI Composites
2.2.1. MWCNTs/EB Composites
0.2 g acid-treated MWCNTs was put into round-bottom flask and then added 10mL deionized water, sonicated for 0.5 h to form uniform suspension, added 4.6 g (0.05 mol) ANI monomer, the reaction mixture was kept in ice bath (−5˚C) with continuous stirring for keeping the temperature constant and stable during reaction. PANI in this form (Emeraldine base (EB)) was synthesized by oxidative chemical polymerization of ANI using ammonium persulfate (APS) at a molar ratio of 1:1. APS aqueous solution (0.05 mol APS dissolved into 50 mL 1 M HCl) was added dropwise in three separate portions with half an hour interval into the above mixture under stirring. The polymerization process was carried out at −5˚C for 6 h with stirring after which it was left overnight. Finally the product was filtered and washed with deionized water and then with ethanol. The polymer was transferred to a 250 mL beaker containing 200 mL of 3% NH4OH, stirred for 12 hours, and subsequently filtered to collect the MWCNTs/EB (EBC) composite powder, and dried in a vacuum oven at 80˚C for 8 hours and stored at room temperature.
2.2.2. MWCNTs/L-PANI (or MWCNTs/D-PANI) Composites
EBC composite powder, which was prepared by a synthetic method mentioned in Section 2.2.1 was added to a 250 mL beaker first, and then 0.06 mol D-CSA (or L-CSA) was added into reaction vessel. The mixture was kept at room temperature with stirring and keeping the temperature constant during reaction. The doping process was carried out for 36 h with continuously stirring. Finally the product was filtered and washed with deionized water and then with ethanol. The prepared MWCNTs/chiral-PANI composites was then dried in a vacuum oven at 80˚C for 8 hours and stored at room temperature.
2.3. Characterization of the Composites
The Fourier transform infrared (FT-IR) spectra of MWCNTs/L-PANI (L-C), MWCNTs/D-PANI (D-C) and EBC composites were obtained using a FT-IR spectrophotometer (Perkin-Elmer, USA, model Spectrum-BX) at room temperature. X-ray diffraction (XRD) measurements using Cu Ka radiation were performed with a powder X-ray diffractometer (RINT 1100, Rigaku). Surface morphology of the composites was observed by transmission electron microscopy (TEM) observations (JEOL-2010, 200 kV) at various magnifications. The samples for TEM measurements were prepared by adding a few drops of ethanol suspension of the composites on carbon mesh-coated copper grids. Thermogravimetric analysis (TGA) was carried out by a SEIKO I & E SSC/570 thermal controller and a TG/DTA 30 at a heating rate of 10˚C/min in N2 atmosphere in the temperature range of 0˚C - 800˚C. A network analyzer HP8720Et was employed to determine the values of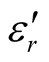 ,
,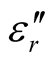 ,
,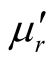 , and
, and  at different frequencies by using a reflection/transmission technique. For this, toroidal-shaped samples of 3 mm inner diameter and 7 mm outer diameter were tightly inserted into a standard coaxial line. Sample lengths approximately equal to one quarter of the guide wavelength were chosen for accurate measurements, so the sample lengths varied from 2 to 3 mm.
at different frequencies by using a reflection/transmission technique. For this, toroidal-shaped samples of 3 mm inner diameter and 7 mm outer diameter were tightly inserted into a standard coaxial line. Sample lengths approximately equal to one quarter of the guide wavelength were chosen for accurate measurements, so the sample lengths varied from 2 to 3 mm.
3. Results and Discussion
3.1. FT-IR Spectroscopy
The FT-IR spectra of L-C, EBC and D-C are shown in Figure 1. The characteristic absorption bands at 1589 and 1499 cm−1 are attributed to C = C stretching of the quinoid and C = C stretching of the benzenoid rings, respectively. Furthermore, the peaks at 1310 and 1160 cm−1 are ascribed to C-N and C-N+• (polaron of PANI) stretching modes, respectively, and the band at 1101 cm−1 represents the C-H aromatic in-plane bending. These bands are in good agreement with the spectrum of PANI when in the form of an emeraldine salt [36] [37] . In addition, the bands at 821 and 583 cm−1 are assigned to the absorption of −SO3H, suggesting that the prepared composites were doped with D-CSA (or L-CSA) [38] . The peak at 1120 cm−1 is assigned to C-O stretching vibration and the peak at 1720 cm−1 is attributed to –C = O stretching vibration in the carboxyl group. This indicates the −COOH group functionalization of MWCNTs [39] [40] .
3.2. XRD
The structural characteristics of EB and composite have been studied by XRD as shown in Figure 2. The 2θ
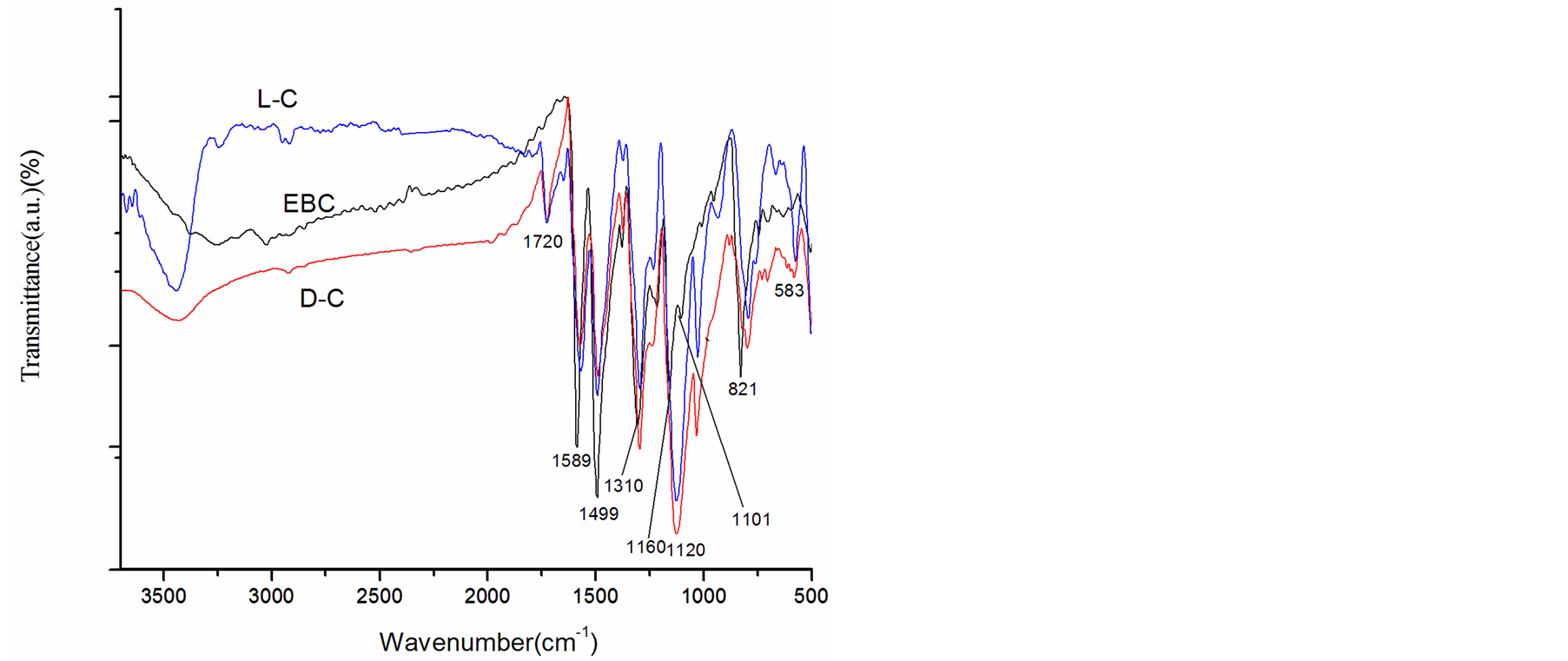
Figure 1. FT-IR spectra of EBC, L-C and D-C composites.
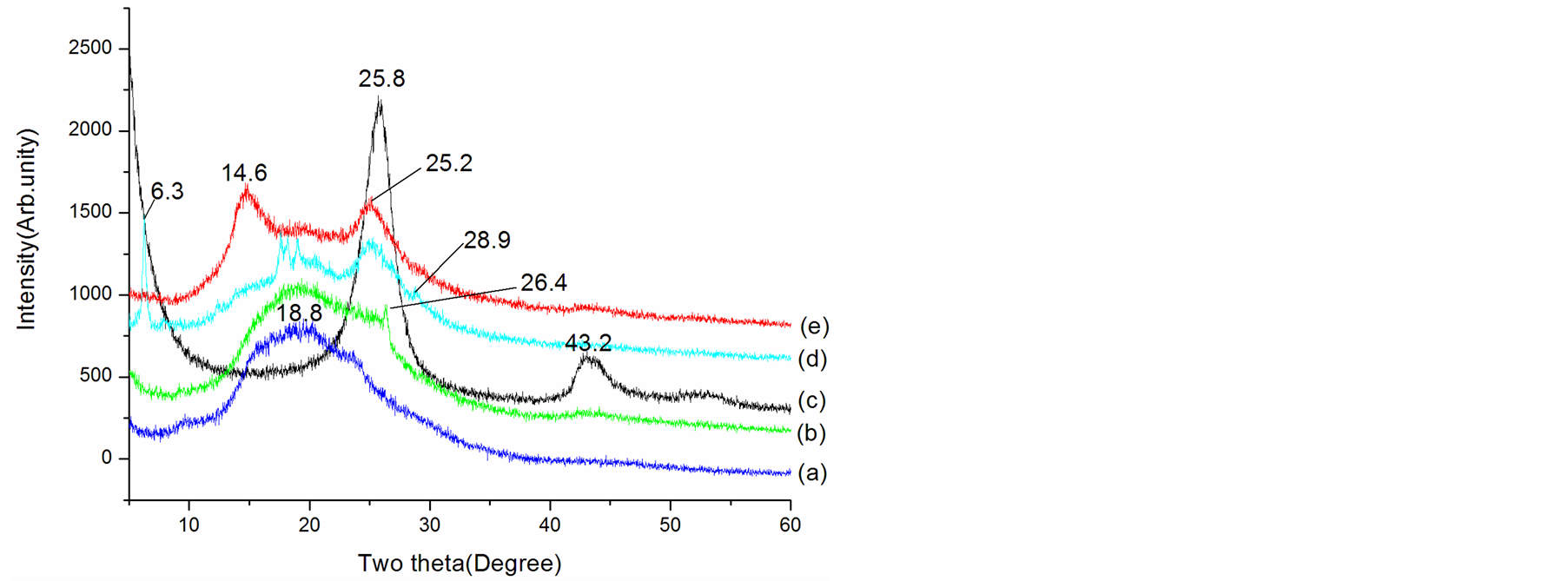
Figure 2. XRD patterns of (a) EB, (b) EBC, (c) MWCNTs, (d) L-C and (e) D-C composites.
scan shows the typical XRD pattern for EB [41] -[44] and MWCNTs [45] , which are shown in Figure 2 noted with a and c, respectively. In the pattern of pristine EB, the XRD pattern exhibits a broad amorphous diffraction peak centered at 18.8˚ indicating that the EB is fully amorphous, which may be ascribed to the periodicity parallel to the polymer chain [44] . For pristine MWCNTs, two characteristic peaks at 2θ = 25.8˚ and 43.2˚ are observed (Figure 2(d)), corresponding to the intershell spacing of the concentric cylinders of graphitic carbon and small amount of catalytic particles encapsulated inside the walls of CNTs [45] [46] . For the EBC composite, the XRD data showed the characteristic EB broad peaks, besides, a small sharp peak at 2θ = 26.4˚ appeared. This peak can be ascribed to the peak of MWCNTs. Compared XRD patterns of L-C and D-C with MWCNTs and EB, it was clear that additional order structures have been introduced in L-C and D-C. The spectra for the D-C clearly reveal new diffraction peak at 2θ = 14.6˚, ones for L-C peaks centered at 2θ = 6.3˚, 17.7˚, 18.3˚ and 19.0˚, it can be seen that the relative intensities of MWCNTs diffraction peaks increase comparing with ones of EBC for the D-C and L-C. The appearance of the new peaks as well as the increase in peak intensity indicates an increase in the extension of microcrystalling domains in the D-C and L-C. These dominant features in D-C and L-C indicate that the formation and stability of the new structures is enhanced when chiral CSA (Dor L-) were used to dope the composites.
3.3. TEM
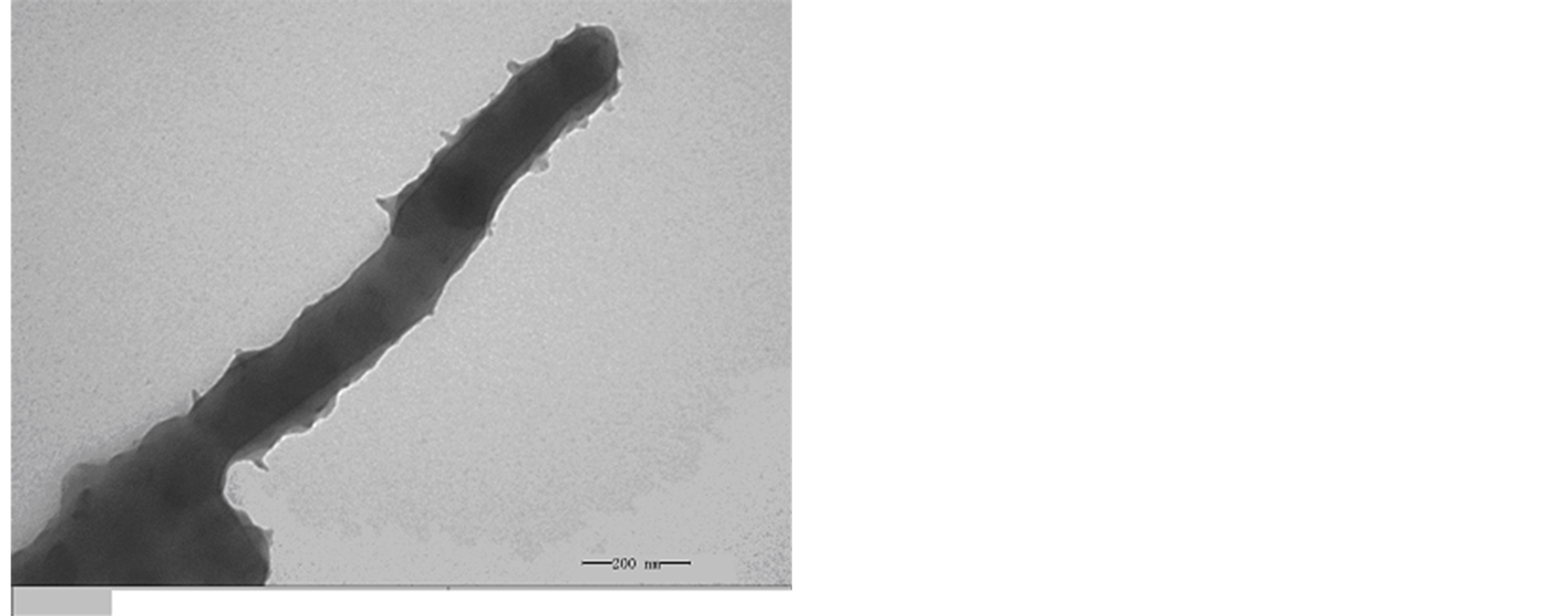
A TEM image of the MWCNTs is shown in Figure 3. The TEM image reveals that the MWCNTs are straight
with diameter 5 - 8 nm, internal diameter 2 - 5 nm and length 10 - 30 μm. It can be seen in Figure 3 that MWCNTs scatter uniformity, and the surface is smooth. Figure 4(a) and Figure 4(b) show the morphologies of L-C. It can be observed that when MWCNTs coated with chiral PANI, the surface of the L-C presents some small spines like sharp needle. The length of the sharp needle is about 40 - 45 nm, the bottom diameter of the sharp needle is about 40 - 43 nm. Compared with the MWCNTs, it can be seen from Figure 4(b) the diameter of the L-C is about 130 - 140 nm, the average thickness of coating layer is about 60 nm (The length of the sharp needle is not included).
3.4. TGA
Thermal stability testing of the composite and PANI were examined by a TGA. In Figure 5 and Figure 6 there are comparisons of the mass losses of PANI, PANI-(L-CSA), PANI-(D-CSA) and EBC composites upon heating in a nitrogen atmosphere. MWCNTs are very stable and no decomposition takes place until 600˚C in nitrogen atmosphere [42] . PANI displays a sudden decrease in mass loss of about 34% at a temperature of 658˚C, and the onset temperature of its TGA curve is 510.5˚C, and the peak temperature is 555.5˚C for the corresponding DTG curve, which is corresponding to the thermal decomposition reaction of PANI and the total mass loss at up to 786˚C can be estimated to be about 40%. Here, the trend of the degradation curve of EBC is similar to that of PANI, the degradation of the EBC composite is mainly controlled by that of PANI. The chiral polyanilie (PANI-(L-CSA) or PANI-(D-CSA)) shows a 26.3% mass loss from 238.93 to 375.96˚C, which is corresponding to the thermal decomposition reaction of doping CSA. After 679.85˚C, another 37.8% mass loss is observed due to the decomposition reaction of PANI, and the total mass loss at up to 786˚C can be estimated to be about 35%.
3.5. Measurement of the Microwave Absorption Effects
Since the absorbing properties of microwave absorbing materials can be estimated from their magnetic and dielectric properties, the essence of researching microwave absorbers is to design the compound and structure form of the materials, by adjusting and optimizing the dielectric and magnetic properties such as complex permittivity and permeability of the materials [47] . In order to investigate the intrinsic reasons for microwave absorption of the composites, we measured the complex permittivity and permeability of all the composites at the frequency range of 2 - 18 GHz. Figure 7 shows the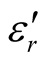 ,
, 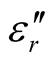 and the dielectric dissipation factor (tanδε =
and the dielectric dissipation factor (tanδε =  /
/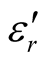 ) vs frequency spectra of the composites, CNTs, and chiral PANI. The
) vs frequency spectra of the composites, CNTs, and chiral PANI. The 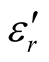 and
and 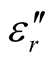 of the L-PANI are 2.65 to3.14 and 0.03 to 0.25 respectively. The highest values of the
of the L-PANI are 2.65 to3.14 and 0.03 to 0.25 respectively. The highest values of the 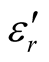 and
and 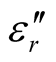 for the D-C composite reach 9.76 and 2.76, respectively. The
for the D-C composite reach 9.76 and 2.76, respectively. The ,
, 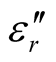 , and tanδε of the L-C composite range from 5.54 to 8.96 and 1.22 to 2.72, and 0.27 to 0.36, respectively. And those of D-C composites are 6.26 to 9.76, 1.14 to 2.76 and 0.22 to 0.31, respectively. And those of the D-PANI are 3.11 to 4.20, 0.30 to 0.70, and 0.09 - 0.21, respectively. As can be seen, the
, and tanδε of the L-C composite range from 5.54 to 8.96 and 1.22 to 2.72, and 0.27 to 0.36, respectively. And those of D-C composites are 6.26 to 9.76, 1.14 to 2.76 and 0.22 to 0.31, respectively. And those of the D-PANI are 3.11 to 4.20, 0.30 to 0.70, and 0.09 - 0.21, respectively. As can be seen, the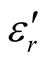 ,
, 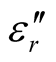 , and tanδε increase dramatically as the addition of MWCNTs in the composites. Figure 8 shows the
, and tanδε increase dramatically as the addition of MWCNTs in the composites. Figure 8 shows the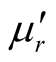 ,
, 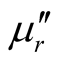 and magnetic dissipation factor (
and magnetic dissipation factor (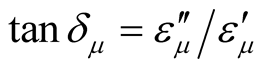 ) vs frequency spectra of the composites, CNTs, and
) vs frequency spectra of the composites, CNTs, and
 (a)
(a)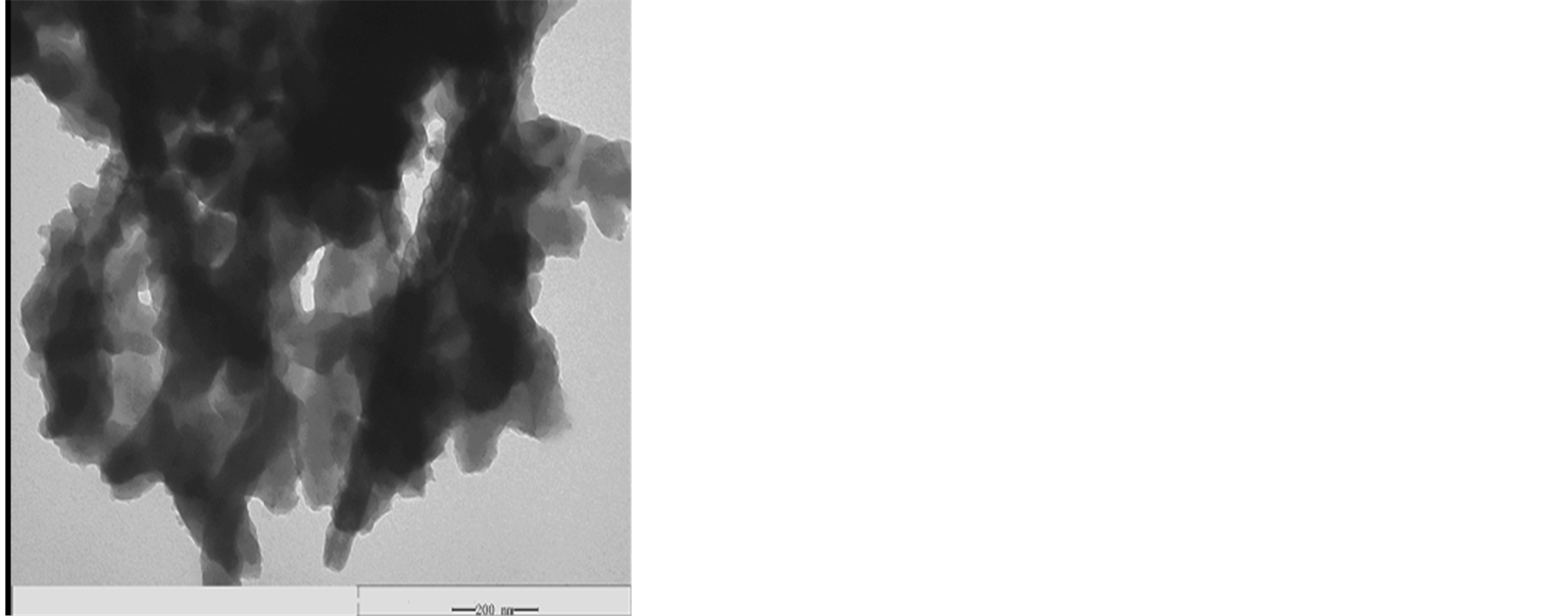 (b)
(b)
Figure 4. TEM images of L-C, (a) L-C, (b) A single L-C thread.
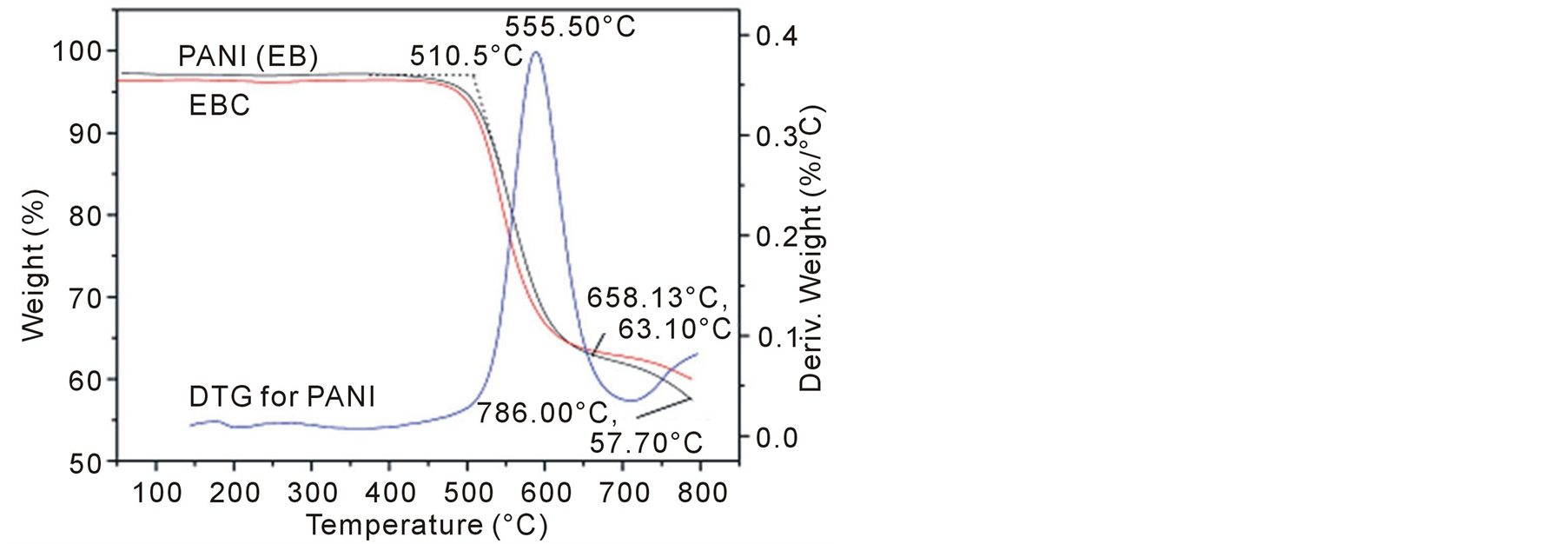
Figure 5. TGA-DTG curve of PANI and TGA curve of MWNTs/ L-PANI at a heating rate of 10℃/min measured under a nitrogen atmosphere.

Figure 6. TGA curves of chiral PANI at a heating rate of 10℃/ min measured under a nitrogen atmosphere.
chiral PANI. As can be seen from Figures 8(a)-(c), the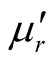 ,
, 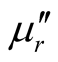 , and
, and 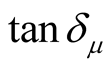 have no large change as the addition of MWCNTs in the composites. The
have no large change as the addition of MWCNTs in the composites. The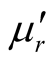 ,
,  , and
, and 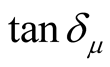 of the L-C composite range from 0.93 to 1.43,
of the L-C composite range from 0.93 to 1.43,
−0.08 to 0.16, and −0.08 to 0.15, respectively. And those of D-C composite are 0.92 to 1.40, −0.92 to 0.19 and −0.10 to 0.16, respectively.
As illustrated in Figure 7 and Figure 8, it is observed that the value of dielectric loss is much higher than that of magnetic loss at the considered frequency. It indicates that the composites have a good and stable dielectric loss property. It suggests that the microwave absorption enhancement of the composites mainly results from dielectric loss, rather than magnetic loss.
To predict the microwave-absorption of all the composites, calculations were carried out to determine the reflection loss. Thus, the reflection loss R could be obtained as following [48] [49] :
The normalized input impedance Zin of a metal-backed microwave-absorbing layer is given by
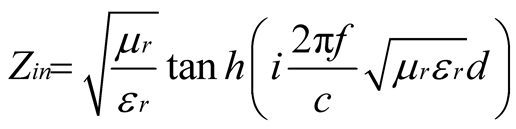 (1)
(1)
where μr (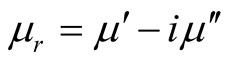 ) and εr (
) and εr (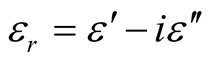 ) are the relative permeability and permittivity, respectively, of the composite medium, c is the velocity of electromagnetic waves in free space, f is the frequency of microwaves, and d is the thickness of the absorber. The reflection loss is related to Zin as:
) are the relative permeability and permittivity, respectively, of the composite medium, c is the velocity of electromagnetic waves in free space, f is the frequency of microwaves, and d is the thickness of the absorber. The reflection loss is related to Zin as:
 (2)
(2)
Thus, the surface reflectance of an absorber is a function of six characteristic parameters, viz., f, d,  ,
, 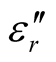 ,
, 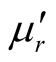 and
and . It is possible to evaluate the numerical values of these parameters corresponding to the condition of zero reflection (Zin = 1).
. It is possible to evaluate the numerical values of these parameters corresponding to the condition of zero reflection (Zin = 1).
Figure 9 shows the frequency dependence of the reflection loss of the composites. It can be seen that the addition of the MWCNTs has an obvious effect on microwave absorbing properties. As seen from Figure 9, the microwave absorbing difference of the chiral PANI between L-PANI and D-PANI is visible. Although the absorption difference between L-C and D-C composite is small, it could be drawn a conclusion that the microwave absorbing property for these materials is related to their chirality. The L-C composite achieved a reflection loss below −10 dB over 1.6 GHz in the range of 5.8 to 7.4 GHz, and the maximum value was −14.95 dB at 6.72 GHz, the reflection loss below −10 dB for the D-C was over 1.8 GHz in the range of 5.2 to 7.0 GHz, and the maximum value was −13.11 dB at 5.92 GHz. And the absorption curve illustrates that the maximum value of reflection loss for the MWCNTs is −8.89 dB at 7.04 GHz, and that for L-PANI and D-PANI are −1.23 dB at 10.32 GHz and −6.34 dB at 10.16 GHz, respectively. When the MWCNTs were coated with PANI, the microwave absorption peaks of the corresponding composites moved to the lower frequency.
4. Conclusion
In conclusion, MWCNTs/chiral-PANI composites have been prepared successfully by in-situ chemical oxidative polymerization of ANI as monomer, L-CSA and D-CSA as chiral doping reagents, APS as oxidative onto

Figure 9. Calculated reflection loss for different composite specimens with maximum attenuation.
MWCNTs. The characterizations of the molecular structure indicate that additional order has been introduced in L-C and D-C composites due to the presence of chiral CSA. The surface of L-C composite presents some small PANI spines with height of about 40 - 45 nm and bottom diameter of about 40 - 43 nm. Finally, the complex permeability, permittivity, and microwave absorption of the composites have been studied. Compared with those of the L-PANI and D-PANI, the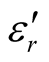 ,
,  and tanδε of the L-C and D-C are much greater, while the
and tanδε of the L-C and D-C are much greater, while the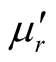 ,
,  and tanδμ are smaller. It is found that the permittivity has remarkable effect on microwave absorption property of the samples, but the magnetization degree and magnetic loss of the samples are small, within the frequency range of 2.0 ~ 18.0 GHz, the D-C composite achieved a reflection loss below −5 dB over 6.1 GHz in the range of 4.1 ~ 10.2 GHz, and the maximum value was −14.95 dB at 6.72 GHz, the reflection loss below −5 dB for the L-C composite is over −4.5 GHz in the range of 4.0 ~ 8.5 GHz, and the maximum value is −13.11 dB at 5.92 GHz. It is concluded that the microwave absorbing property of the chiral composites is function of its chirality.
and tanδμ are smaller. It is found that the permittivity has remarkable effect on microwave absorption property of the samples, but the magnetization degree and magnetic loss of the samples are small, within the frequency range of 2.0 ~ 18.0 GHz, the D-C composite achieved a reflection loss below −5 dB over 6.1 GHz in the range of 4.1 ~ 10.2 GHz, and the maximum value was −14.95 dB at 6.72 GHz, the reflection loss below −5 dB for the L-C composite is over −4.5 GHz in the range of 4.0 ~ 8.5 GHz, and the maximum value is −13.11 dB at 5.92 GHz. It is concluded that the microwave absorbing property of the chiral composites is function of its chirality.
NOTES
*Corresponding author.