The Possible Role of the Incretin Enhancer Sitaglipten, in Renal Ischemic Reperfusion Injury in Type 2 Diabetes Mellitus ()
1. Introduction
Type 2 diabetes mellitus or non-insulin-dependent diabetes mellitus (NIDDM) is one of the leading causes of end-stage renal disease (ESRD) [1] . Diabetic patients are at a higher risk of ischemic changes compared to nondiabetics [2] . With increasing duration and severity of ischemia, greater cell damage can develop [3] [4] . The mechanisms behind the injury in diabetic nephropathy are not fully understood despite intense researches. Diabetic patients may need renal transplantation in their later life due to diabetic nephropathy. The short period of ischemia (30 min) in diabetes has been demonstrated to lead to reversible renal failure, however progressive injury lead to ESRD [4] . Reactive oxygen species (ROS) and nitric oxide (NO) play an important role in mediating cell damage during ischemia-reperfusion (I/R) injury [5] . Inflammation contributes substantially to the pathogenesis of I/R with a central role for particular cells, adhesion molecules, and cytokines [6] . Neutrophils produce abundantly ROS during I/R injury. Myeloperoxidase (MPO) is found in neutrophils, which catalyze the formation of hypochlorous acid (HOCl), a toxic agent to cellular components, and initiates oxidative injury [7] . Renal I/R injury may cause oxidative stress and increase lipid peroxidation in the tissue.
Although many antidiabetic drugs have been developed and used for treatment so far, current treatment strategies for type 2 diabetes have only limited long-term efficacy and tolerability given the progressive nature of the disease [8] . As such, there is a need to develop efficient new therapeutic strategies for the treatment of type 2 DM. Primary defects of insulin action (insulin resistance), along with a defect of insulin secretion, contribute to the etiology of this type of diabetes. Diminished postprandial insulin secretion resulting from both functional defects and the loss of surviving pancreatic β-cells is known to progress into hyperglycemia and a decline in insulin sensitivity [9] . Of the currently available oral antidiabetic agents, sulfonylureas have a superior postprandial antihyperglycemic effect due to their potent insulinotropic action. These agents are widely used by many diabetic patients, and there are few primary nonresponders [10] [11] . However, sulfonylureas potently and persistently stimulate insulin secretion irrespective of blood glucose levels, thereby causing hypoglycemia, which is a common undesirable side effect [12] .
During the investigation of new antidiabetic drugs that do not have the disadvantages of sulfonylureas, glucagon-like peptide 1 (GLP-1), an incretin analogue, attracted attention. GLP-1, which is a 30 amino acid hormone secreted from L cells of the small intestine and proximal colon in response to the ingestion of nutrients, promotes insulin secretion through GLP-1 receptors expressed on pancreatic β-cells [13] . In addition, the insulinotropic action of GLP-1 is glucose-dependent; therefore, the risk of hypoglycemia is minimized [14] . However, the therapeutic use of GLP-1 is severely compromised by its lack of oral activity as well as its rapid degradation and inactivation by dipeptidyl peptidase (DPP)-IV, a serine protease that is present in soluble form in body fluids and is also expressed in many tissues, including the kidney and liver [15] . Thus, DPP-IV inhibitors, which prevent the proteolytic degradation of GLP-1 and enhance glucose-dependent insulin secretion from pancreatic β-cells, are a useful new class of antidiabetic drugs with a lower risk of inducing hypoglycemia than conventional sulfonylureas. Furthermore, clinical efficacy has been reported for DPP-IV inhibitors such as sitagliptin, vildagliptin, and saxagliptin [16] -[18] . GLP-1 receptor agonist (exenatide) has reported efficacy in ischemia reperfusion (I/R) injury [19] [20] .
Aim: So far, little is known regarding the effect of sitaglipten on renal I/R in type 2 DM, and therefore, this study was designed to investigate its effect on renal I/R in diabetic rats.
2. Materials and Methods
2.1. Chemicals
Superoxide dismutase (SOD), crystalline beef liver catalase (CAT), 1, 1, 3, 3-tetrahydroxy-propane, glutathione (GSH), and epinephrine hydrochloride. Ethidium bromide, agarose, tris buffer, thiobarbituric acid, trichloroacetic acid, and Folin’s phenol reagent. The diagnostic kits were used for estimation of BUN and creatinine. All other chemicals used in the study were of laboratory grade.
2.2. Experimental Groups and Animals
Experimental Animals
Fifty adult male albino rats weighing 250 ± 10 g were used in this study. All rats had free access to standard chow diet and tap water. They were housed in metabolic cages at 20˚C - 25˚C and kept under constant conditions with 12/12 h light/dark cycles and left for acclimatization for one week before the start of the study. The care and handling of the animals were in accordance with the guidelines of the National Institutes of Health (NIH).
The rats were divided into five different groups, each of 10 rats (n = 10).
Group-1: Normal sham-operated (underwent all surgical procedures without I/R in normal rats).
Group-2: Diabetic sham-operated (underwent all surgical procedures without I/R in diabetic rats).
Group-3: (I/R control), in normal rats; on day 28, ischemia was produced for 30 min, followed by reperfusion for 24 hours (hrs)
Group-4: (DM + I/R control), after induction of diabetes; on day 28, I/R was produced.
Group-5: (Sitaglipten treated group + I/R), in diabetic rats; on day 14, rats were given sitaglipten (10 mg per kg of body weight per day, P.O) [21] (Sigma, St.Louis, MO) then, on day 28, I/R was produced (Figure 1).
2.3. Methods
2.3.1. Induction of DM and Measurement of Blood Glucose Level
Diabetes mellitus in rats were induced by administration of nicotinamide (NIC) (230 mg/kg, i.p.), 15 min prior to a single intraperitoneal (i.p.) injection of streptozotocin (65 mg/kg, STZ) dissolved in di-sodium citrate buffer (pH 4.5) in a dose volume of 1 ml/kg body weight [22] . Control animals were received an equal volume of saline. After one week following NIC and STZ administration, blood was collected from tail vein after overnight fasting rats for 16 hrs, and serum samples were analyzed for blood glucose(by using standard diagnostic kits). Animals showing fasting blood glucose higher than 250 mg/dl were considered as diabetic and used for the further study. Four weeks elapsed in between the induction of diabetes and ischemic injury.
STZ &NIC and sitaglipten were purchased from (Sigma Chemical Company Cairo, Egypt). Sitaglipten was given orally once a day by gastric tube at a dose of 10 mg/kg and was dissolved in distilled water and given orally for diabetic rats for two weeks. A similar volume of water was given to the other four groups orally once a day by gastric tube for two weeks (Placebo).
2.3.2. Renal I/R Injury
Diabetic and normal rats were anesthetized with ketamine (60 mg/kg i.p.) and diazepam (5 mg/kg i.p.). After anesthesia, the animal was fixed in supine position on the operating table and the abdominal skin was shaved and disinfected with povidone-iodine solution. All rats were undergoing surgical exposure of the left and right renal pedicles via midline incision. To induce renal ischemia, both renal pedicles were occluded for 30 min using a non-traumatic vascular clamp (Bulldog clamp). After removal of the vascular clamp, the abdomen was properly irrigated with isotonic saline, and then the abdominal incision was closed by continuous stitches using vicryl 2/0 sutures.
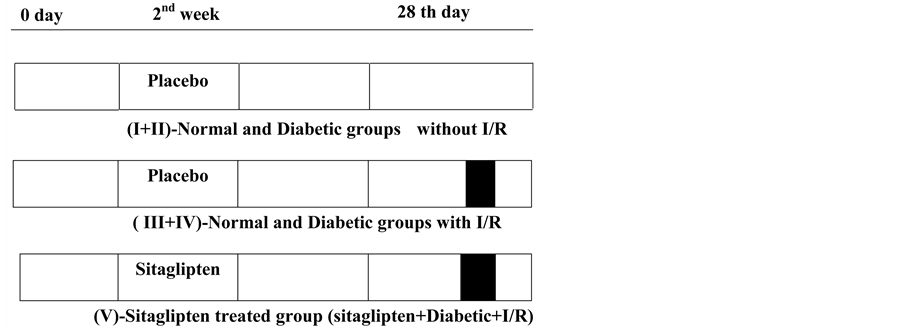
Figure 1. Scheme representing the experimental protocols employed. Each protocol comprised the following periods: treatment period (Placebo or sitagliptin) 14 days before induction of ischemia. Ischemia for 30 min and reperfusion for 24 hrs.
2.3.3. Collection of Blood Samples
Blood samples were taken (24 hrs after ischemia) using capillary tubes introduced into the medial retro-orbital venous plexus. Sera were separated from clotted blood after 20 - 30 min by centrifugation at 4000 rpm for 15 min then divided into aliquots and kept at −20˚C for assay of blood urea nitrogen (BUN) (Jaffe’s method), and creatinine (DAM method) by using standard diagnostic kits, while serum glucose was determined immediately.
2.3.4. Determination of Serum Glucose, BUN, Creatinine, and TNF-α
Serum glucose was determined by using diagnostic kits (Spinreact, Spain), following the manufacturer instructions. BUN was estimated by using commercial kits (Spinreact, Spain), following the manufacturer instructions. Serum creatinine was determined by using auto analyzer apparatus (CX7, Beckman, USA). Serum TNF-α was determined by using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions.
2.3.5. Harvesting of Kidney Specimen
Kidney specimens were harvested 24 hrs after ischemia. Animals were anaesthetized with high dose of sodium thiopental i.p. Then, abdomen was rapidly opened and kidneys were removed rapidly, one was placed in 10% neutral buffered formalin for H and E staining and the other one was rapidly frozen in liquid nitrogen, and stored at −70˚C until tested.
2.3.6. Lipid Peroxidation and Antioxidant Enzymes
The kidney was cross-chopped with surgical scalpel into fine slices in chilled 0.25 M sucrose, quickly blotted on a filter paper. The tissue was minced and homogenized in 10 mM Tris-HCl buffer, pH 7.4 (10% w/v) with 25 strokes of tight Teflon pestle of a glass homogenizer at a speed of 2500 rpm. The clear supernatant was used for assays of lipid peroxidation (MDA content) and endogenous antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), and glutathione peroxidase (GSHPx). The MDA formation was estimated by the method of Slater and Sawyer [23] . Reduced GSH was determined by the method of Moran et al. [24] . SOD was determined by the method of Mishra and Fridovich [25] . Catalase was estimated by the method of Hugo Aebi as given by Colowick et al. [26] . GSH peroxidase was determined by the method of Paglia and Valentine [27] .
2.3.7. Xanthine Oxidase Activity
Tissue xanthine oxidase (XO) activity was measured spectrophotometrically by the formation of uric acid from xanthine through the increase in absorbance at 293 nm [28] .
2.3.8. Nitric Oxide Level
The nitric oxide (NO) was estimated by the method of Lepoivre et al. [29] .
2.3.9. Myeloperoxidase Activity (MPO)
MPO activity was measured in tissues in a procedure similar to that documented by Hillegas et al. [30] .
1) Renal Histopathology The kidney was fixed in 10% neutral-buffered formalin solution, embedded in paraffin, and was used for histopathological examination. Sections of 5 μm thickness were cut on a microtome and taken on glass slides coated with albumin. The hematoxyline-stained sections were stained with eosin for 2 min and were then quickly passed through ascending grades of alcohol, cleaned in xylene and mounted on Canada Balsam. The stained sections were examined under Olympus BX40 photomicroscope and photographed, to determine histopathological changes. A minimum of 10 fields for each kidney slide were examined and assigned for severity of changes using scores on a scale of none (−), mild (+), moderate (++), and severe (+++) damage [31] .
2) Statistical Analysis [32]
Data were expressed as means with their corresponding standard deviations (SD). One way analysis of variance (ANOVA or F-test) was used to compare more than two means. Then the data were subjected to least significant difference (LSD) test.
3. Results
3.1. Effect of Sitaglipten on Renal Function (Table 1, Figure 2, Figure 3 & Figure 4)
Diabetic animals that underwent renal I/R exhibited significant increase in the serum concentrations of urea nitrogen and creatinine, as compared to I/R animals, suggesting a significant degree of glomerular dysfunction mediated by diabetes. Pretreatment of rats with sitaglipten (10 mcg/kg) produced, a significant reduction in the serum levels of these parameters as compared to DM + I/R group (Table 1, Figure 2, Figure 3 & Figure 4).
3.2. Effect of Sitaglipten on Lipid Peroxidation (Table 2, Figures 5, 6, 7, 8, 9) and Antioxidant Enzymes (Table 3, Figures 10, 11, 12, 13)
The serum TNF-α level was significantly increased in the I/R control as compared to normal control group. In DM + I/R rats, serum TNF-α level was significantly higher as compared to I/R control. Sitaglipten treated group had significantly lower serum TNF-α level as compared to the DM + I/R control group (Table 2, Figure 5).
The renal tissue MDA content in the normal as well as in diabetic group was elevated after induction of I/R injury, compared to normal control group. However, sitaglipten treated group, significantly decreased the I/R-induced elevation in renal MDA level as compared to DM + I/R group (Table 2, Figure 6).

Table 1. Effect of oral administration of sitaglipten on blood urea nitrogen (BUN), creatinine and serum glucose levels in diabetic ischemic reperfused rats.
a: Significant with normal control group; b: significant with diabetic group; c: Significant with ischemic reperfused (IR) group; d: Significant with diabetic + I/R group; e: Significant with Sitaglipten treated group.
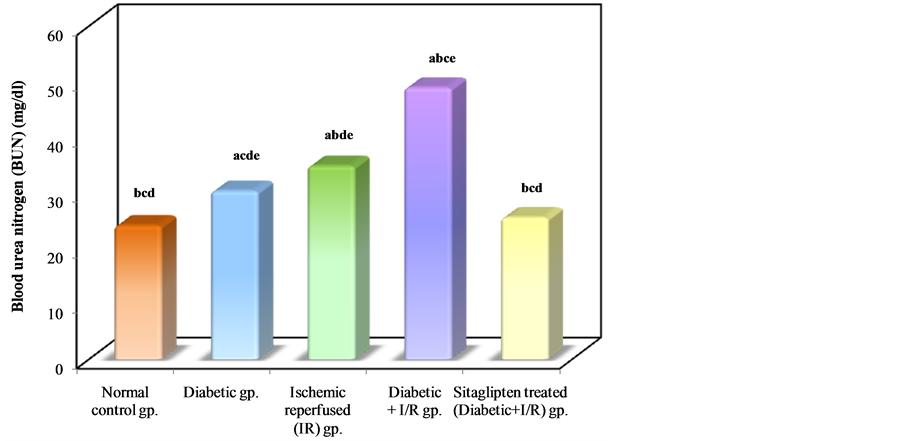
Figure 2. Comparison between the different studied groups according to BUN.
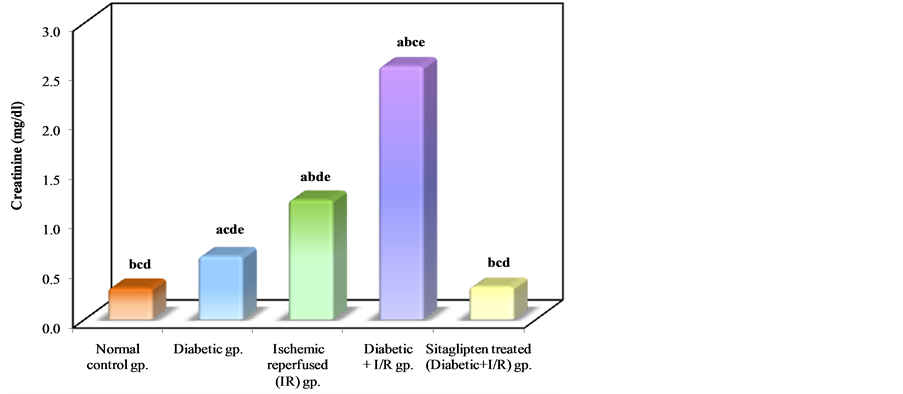
Figure 3. Comparison between the different studied groups according to creatinine.

Figure 4. Comparison between the different studied groups according to serum glucose.

Table 2. Effect of oral administration of sitaglipten on serum tumor necrosis factor-alpha (TNF-α), malondialdehyde (MDA), xanthine oxidase (XO) , nitric oxide (NO) and Myeloperoxidase activity (MPO) in diabetic ischemic reperfused rats.
a: Significant with normal control group; b: significant with diabetic group; c: Significant with ischemic reperfused (IR) group; d: Significant with diabetic + I/R group; e: Significant with Sitaglipten treated group.
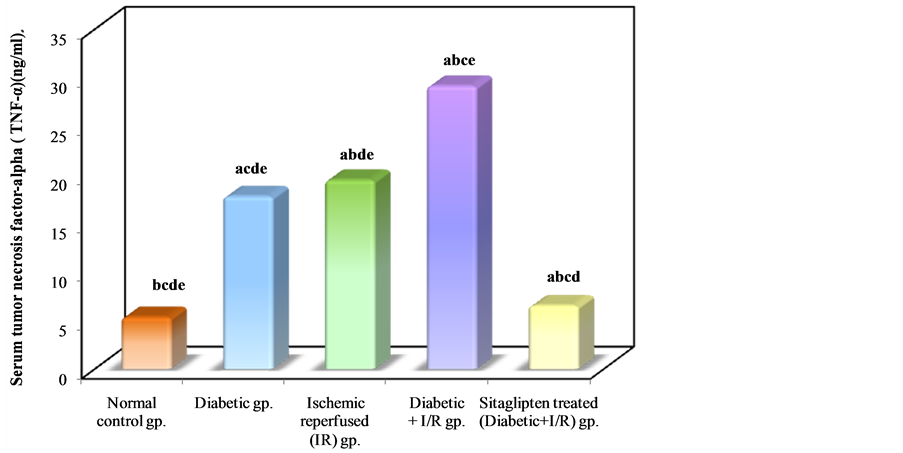
Figure 5. Comparison between the different studied groups according to TNF-α.

Figure 6. Comparison between the different studied groups according to MDA.
The XO activity was increased in I/R and DM + I/R groups as compared to the normal control group. The XO activity was normalized by treatment with sitaglipten compared to DM + I/R (Table 2, Figure 7).
The levels of NO were increased in I/R and DM + I/R groups as compared to normal control group. Sitaglipten treated group demonstrated a significant decrease in NO level as compared to DM + I/R group (Table 2, Figure 8).
Myeloperoxidase (MPO) activity, which is accepted as an indicator of neutrophil infiltration, was significantly higher in the kidney tissue of the DM + I/R group than that of the I/R group. On the other hand, sitaglipten treated group significantly decreased renal tissue MPO level compared to DM + I/R (Table 2, Figure 9).
As regards the antioxidant enzyme activity: Diabetic animals that underwent renal I/R exhibited significant decrease in the SOD activity as compared to I/R animals. However, the activity of SOD in renal tissue was increased in the group pretreated with sitaglipten, as compared to the DM + I/R (Table 3, Figure 10). The catalase (CAT) activity of DM + I/R group was decreased in comparison with I/R group, whereas it was higher in the group

Figure 7. Comparison between the different studied groups according to XO.
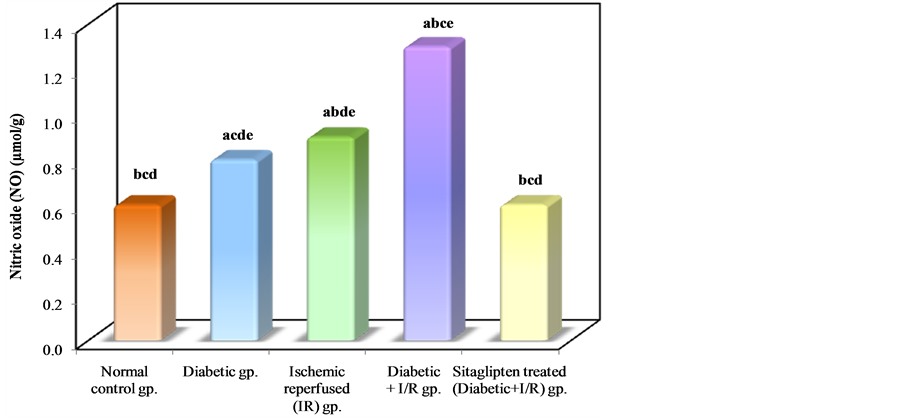
Figure 8. Comparison between the different studied groups according to NO.

Table 3. Effect of oral administration of sitaglipten on superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and glutathione peroxidase (GSHPx) in diabetic ischemic reperfused rats.
a: Significant with normal control group; b: significant with diabetic group; c: Significant with ischemic reperfused (IR) group; d: Significant with diabetic + I/R group; e: Significant with Sitaglipten treated group.
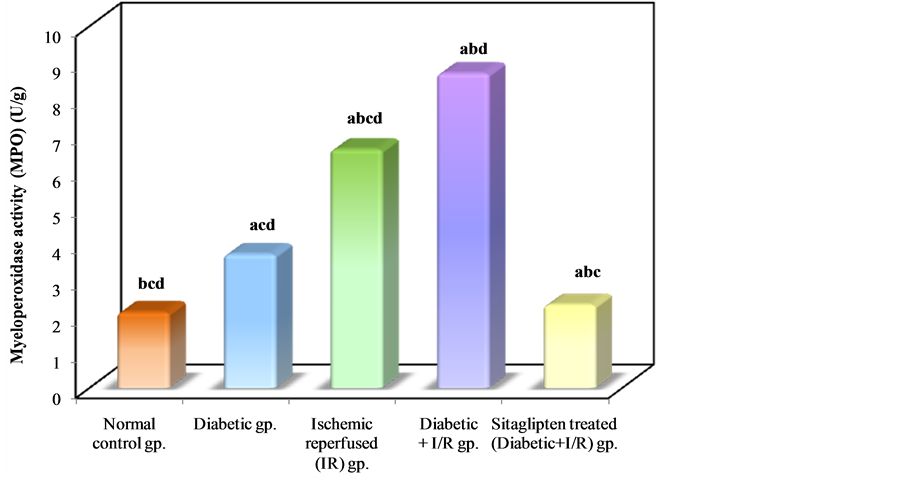
Figure 9. Comparison between the different studied groups according to MPO.
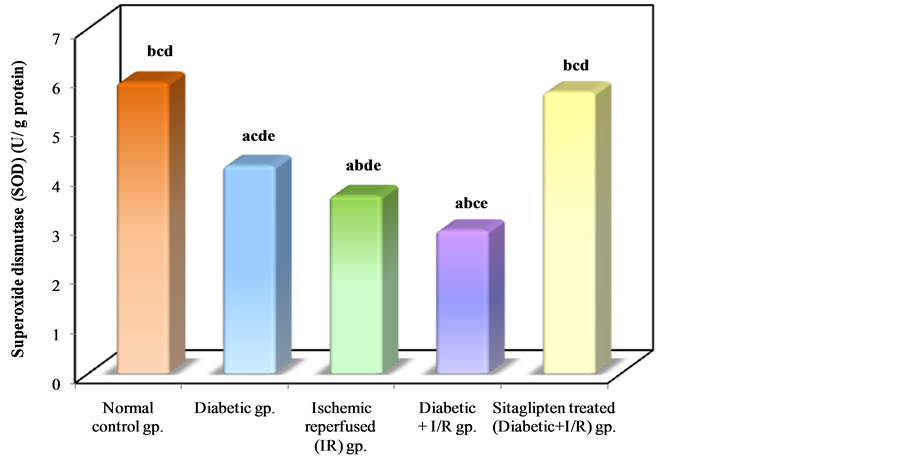
Figure 10. Comparison between the different studied groups according to SOD.
pretreated with sitaglipten than in the DM + I/R group (Table 3, Figure 11).
In I/R diabetic rats ,there was a significant decrease in renal tissue GSH and GSHPx level when compared to normal control group, while in the sitaglipten-treated group, renal GSH, and GSHPx content were found to be preserved (Table 3, Figure 12 & Figure 13).
3.3. Effect of Sitaglipten on Renal Histology
The histopathological changes were shown in (Figure 14). The kidneys of untreated I/R rats showed tubular cell swelling, interstitial edema, tubular dilatation, hyaline casts, and moderate-to-severe necrosis. Treatment with sitaglipten (10 mcg/kg) preserved the normal morphology of the kidney, and shows normal glomeruli and normal tubular cells.
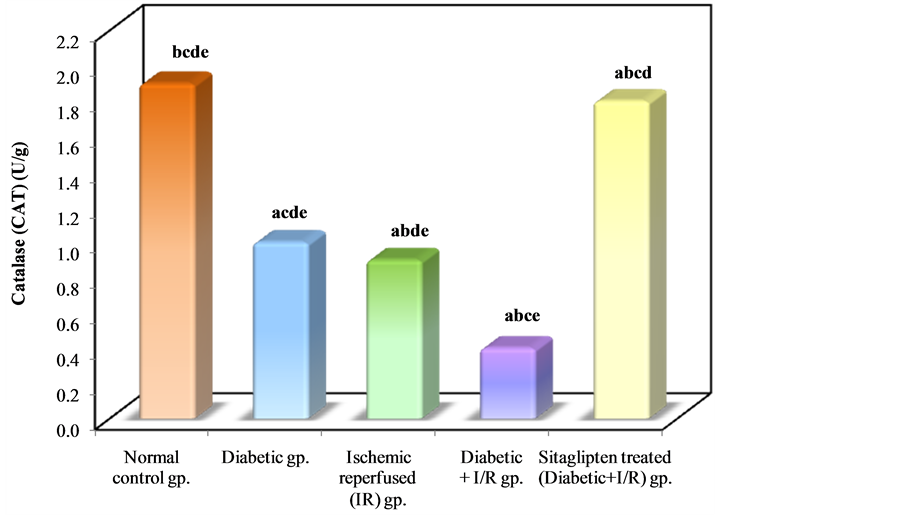
Figure 11. Comparison between the different studied groups according to CAT.
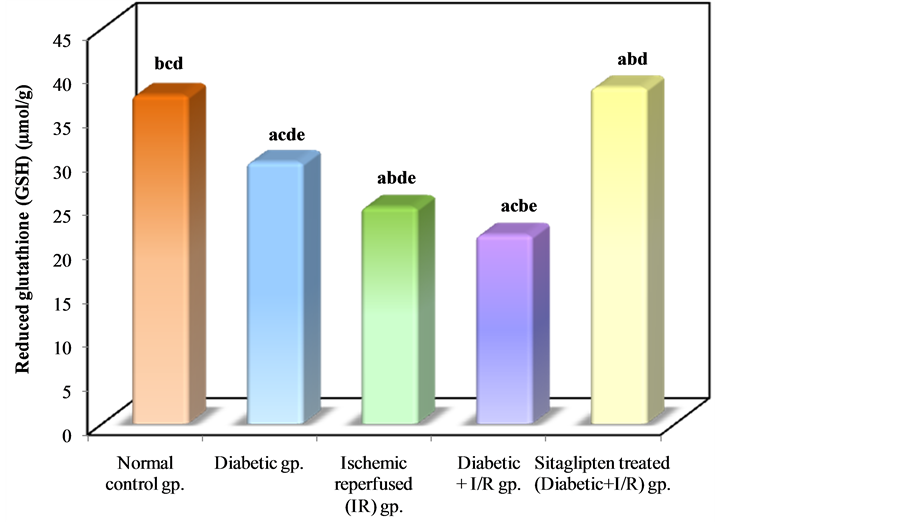
Figure 12. Comparison between the different studied groups according to GSH.
4. Discussion
Diabetes mellitus (DM) especially type 2 causes organ dysfunction and increases the sensitivity of organs to damages. In the present study, we tested the hypothesis that DM and hyperglycaemia render the kidney more susceptible to ischemic injury and examined the effect of renal ischemia for 30 min on renal functions, histological damage, proinflammatory cytokines TNF-α as well as oxidant markers in STZ induced type 2 DM. Also, the present study examined the possible role of sitaglipten in these changes.

Figure 13. Comparison between the different studied groups according to GSHPx.

Figure 14. Sitaglipten improves renal tissue ischemia. Sections were stained with hematoxylin and eosin. (A) Section from normal kidney demonstrates the presence of 3 glomeruli exhibiting round configuration, normal cellularity (red arrow), and normal tubules with healthy lining epithelium (blue arrow head). (10×) (B) Section from diabetic kidney shows thickening of the glomerular capillary basement membrane. (10×) (C) Section from kidney subjected to I/R shows golmerular atrophy (red arrow), influx of inflammatory cells (blue arrow), and necrosis of the epithelial lining of renal tubules (green arrow). (20×) (D) Section from diabetic kidney subjected to I/R Shows marked vascular congestion (red arrow), atrophy and necrosis of the tubular lining epithelium. (20×) (E) Section from diabetic kidney subjected to I/R and treated with sitaglipten (10 mcg/kg) shows that sitaglipten preserved the normal morphology of the kidney, normal glomeruli and normal tubular cells. (10×)
Diabetic rats in the present study showed significant increase in the serum blood glucose, creatinine, blood urea nitrogen, tumor necrosis factor-alpha (TNF-α), malondialdehyde (MDA), xanthine oxidase (XO), nitric oxide (NO) and myeloperoxidase activity (MPO). These markers became significantly higher in diabetic rats after the episode of ischemia. At the same time, there is a negative correlation between sitaglipten and these parameters. These findings suggest the possible role of proinflammatory cytokines; TNF-α and oxidative markers in pathogenesis of ischemic injury in diabetic kidney.
Regarding the increased creatinine and BUN in this study, which are in agreement with several studies which reported that, in experimentally diabetic rats, there is a significant increase in serum creatinine levels ascompared to normoglycemic ones and this is considered as an indicator of deteriorated renal function [33] -[35] . Hyperglycaemia is most probably a contributing factor in the development of renal ischemia. Shortage of insulin could also be involved in the increased sensitivity to I/R. Secondary effects of hyperglycemia such as formation of advanced glycosylated end products (AGEs), increased oxidative stress, hemodynamic alterations, and formation of NO could also be involved.
In addition, our results confirmed the findings of Yousef et al. [36] who found that combination of renal ischemia with DM raised serum creatinine more than did DM alone suggesting significant impairment of glomerular function.
In consistence with renal function impairment, histopathological examination revealed significant increase in acute tubular necrosis score in diabetic ischemic rats with characteristic morphological changes, such as tubular cell swelling, tubular dilatation, necrosis of epithelium, and interstitial edema. In contrast, sections of the sitaglipten pretreated kidneys demonstrated architectural and cytological preservation of structure.
The present study showed significant rise in serum TNF-α level in diabetic non-ischemic, ischemic, and diabetic ischemic rats, as compared to normal control rats, but the marked rise was found in diabetic ischemic rats. These findings are in consistent with many studies that demonstrated significant rise in serum TNF-α level in renal I/R injury [37] -[39] .The increased TNF-α in ischemic diabetic rats may result from constitutive overproduction by adipose tissue in type 2 diabetes, the effects of hyperglycemia and AGEs and increased generation of ROS in renal I/R injury and DM [40] [41] . Hyperglycemia induced oxidative stress, along with AGEs and products of lipid peroxidation, possibly serves as activators of transcription factors, leading to induction of gene expression of pro-inflammatory cytokines and release of many inflammatory cytokines as TNF-α and IL-6 through NF-κB [42] . TNF-α can induce renal injury via induction of apoptosis and necrotic cell death, alterations of intra-glomerular blood flow and glomerular filtration rate as a result of the hemodynamic imbalance between vasoconstrictive and vasodilator mediators, alterations of endothelial permeability, as well as alterations of the barrier function of the glomerular capillary wall leading to enhanced albumin permeability [43] . At the molecular level TNF-α augments the release of many inflammatory factors from renal mesangial cells [44] [45] .
This may confirm our results in which we found that there is a significant positive correlation between serum TNF-α and renal tissue content of MDA and XO.
Another point of interest in the present study was to investigate the involvement of NO in development of renal I/R injury in DM. NO was significantly elevated in renal tissue in diabetic ischemic rats. This result coupe with many authors who documented that iNOS is activated in the kidney of rats, soon after the induction of diabetes, suggesting its involvement in the increased production of NO observed immediately after the onset of diabetes [33] [46] .
The NO system may be involved in the increased sensitivity to I/R in DM. There is evidence for increased NO-production in the STZ-DM kidney, the reaction of NO with O2 results in peroxynitrite formation, a potent and aggressive cellular oxidant and causes the formation of 3-nitro-l-tyrosine. Nitrite/nitrate levels, as the end products of NO conversion, were increased in blood plasma and aortic tissue in diabetic animals as compared to nondiabetic ones [46] which was confirmed by elevated NO level in our study.
Infiltration of inflammatory cells is one of the main features of renal I/R injury in diabetic rats. The infiltrate mainly consisted of cells identified as macrophages/monocytes and T-lymphocytes [47] . This study demonstrated high renal MPO activity after induction of I/R in diabetic rats. This reflects high-leukocyte infiltration in the renal tissue. The neutrophils play a major role in oxidant injury via the mechanisms such as the action of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase or MPO system. HOCl which is produced largely from stimulated neutrophils by MPO activity, causes oxidation of other molecules such as proteins, amino acids, carbohydrates, nucleic acids, and lipids expanding renal tissue damage [48] .
Sitaglipten in this study reduced the oxidative stress and the biomarkers of the inflammation which are determinant factors in the course of renal disease. Treatment with sitaglipten prevented renal I/R-induced lipid peroxidation and protected the kidneys from severe attenuation of antioxidant enzymes activity in rats exposed to the renal I/R. In addition, it improved the functional renal parameters as BUN and creatinine. The histological specimens has proved that sitaglipten treated rats was protected against renal I/R in diabetes.
Several mechanisms might be responsible for the renoprotective effects of sitaglipten against renal I/R. First, the restoration of normoglycemia, because glucose metabolism is stimulated over fatty acid metabolism, which is more efficient with respect to oxygen consumption for adenosine triphosphate production and which might therefore reduce oxygen demand. An increased acute sensitivity to ischemia has been demonstrated when blood glucose level was raised by dextrose infusion or intraperitoneal glucose injection in combination with renal I/R in both rats and dogs [49] . In our study, glucose controlled by sitaglipten pretreatment could has a protective effect against renal I/R in type 2 DM.
Additionally, sitaglipten might reduce apoptosis and oxidative stress. Ferreira et al. demonstrated that the activity of the antioxidant enzymes SOD and catalase were higher in animals treated with sitaglipten and nuclear oxidative stress was reduced. In addition, they demonstrated beneficial effects of sitagliptin on metabolic profile and reduction in inflammatory markers, as well as amelioration of fibrosis, vacuolization, and congestion in endocrine pancreas and preservation of pancreatic islets [50] . Our results agree with those performed by others, which have been suggesting an antioxidant and anti-inflammatory effect of incretin modulators, due to attenuation of the deleterious effects of AGEs-RAGE-oxidative stress axis and to protection against the cytokineinduced apoptosis and necrosis [51] -[53] .
5. Conclusion
In conclusion, type 2 diabetes provoked an exaggerated renal I/R injury in STZ treated rats. Sitaglipten treatment attenuated acute I/R-induced renal injury in diabetic rats modifying the oxidative stress and the inflammation processes. Therefore, the protection afforded reflects both improved glucose metabolism as well as the antiinflammatory, anti-oxidant, anti-fibrotic, and anti-apoptotic effects.
Acknowledgements
The authors gratefully acknowledge the grant of center of scientific research and revival of Islamic heritage No (43209012).
Abbreviations
DM: Diabetes mellitus ESRD: End stage renal disease I/R: Renal ischemia/reperfusion injury
NOTES
*Corresponding author.