1. Introduction
Conditions for plant establishment, growth and yield in arid and semi-arid areas are harsh, because these areas are subjected to natural and anthropogenic stresses. Of these, drought and soil salinity are the main plant growth limiting factors (Shannon, 1998; Mitlöhner, 1998; Serrano et al., 1998). Environments under primary salinity, i.e. climate and soil induced salinity, are covered with adapted vegetation that evolved to cope with the limiting factors of drought and excess salt (Mitlöhner & Koepp, 2007). Saline growth medium affects plant growth due to low osmotic potentials of soil solution (osmotic stress), specific ion effects (salt stress), nutritional imbalance or a combination of these factors (Ashraf, 2004). Climate change is expected to cause temperature rise and shortage of water in most parts of Africa, which will exacerbate high solute concentration. Hence, under such conditions, the physiological regulation of water use in response to water depletion and high solute concentra- tion (salinization) is essential for species survival, productivity, distribution and competitive relationships.
The identification of suitable plant characters, with the aim of selecting the right species to the specific envi- ronment, is indispensible in revegetation and land reclamation efforts. A number of studies have described morphological and physiological adaptive responses displayed by the natural inhabiting vegetation in response to water deficit (e.g. Sobrado, 1986; Sperry & Hacke, 2002). However, such studies have rarely been carried out in the savannah woodlands of Ethiopia, where various economically, socially and environmentally valuable plant species, characterised as being drought and salt tolerant, co-exist. One such species is the evergreen tree, Dobera glabra (Forssk.) Poir. (Salvadoraceae). The fruits of this species ripen during the drought period, thus, making the species particularly useful as a source of food and many other purposes (Tsegaye et al., 2007; Te- ketay et al., 2010). Besides, Farmers and pastoralists in southern Ethiopia, e.g. lowlands of Konso (southern Ethiopia), and Afar (northeastern Ethiopia), use the species as one of the drought indicator plants. This is based on their long-standing observations and experiences of enhanced production of new shoots, fruits and seeds by the species when rains are delayed or fail. As a result, people in Konso and Afar region predict the onset of a drought period and, hence, shortage of food, when the trees bloom abundantly. Except for a few attempts that have studied the influence of grazing and moisture-related factors on woody species recruitment (Tsegaye et al., 2007; Tsegaye et al., 2010), studies about the plant water relations of D. glabra under field conditions are lack- ing. Hence this study examined the plant water relations of D. glabra and co-occurring species with the aim of examining their drought stress tolerance and comparative advantages. To investigate response to drought stress, we measured plant water potential. The water potential parameters commonly used to estimate the extent of wa- ter stress by plants are midday (Ψmd), predawn (Ψpd) and diurnal range (ΔΨ) water potentials (Vertovec et al., 2001; Gebrekirstos et al., 2006). Water potential is rapidly measured in the field and provides a reliable measure of plant and soil water status (Slatyer, 1967; Kramer & Boyer, 1995; Mitlöhner, 1998), particularly for plant comparisons (Vertovec et al., 2001; Gebrekirstos et al., 2006).
To investigate plant reactions towards osmotic stress (site salinity), we applied freezing point osmometry. As the osmotic potential within a plant is comprised of ionic and non-ionic osmotica in different combinations and ratios (Rhodes et al., 2002) electrical conductivity (EC) is not applicable for plants since it exclusively targets the ionic osmotica in a solvent (Mitlöhner & Kopp, 2007). However, within the plant the non-ionic osmotica, i.e. glycine betaine, proline and sucrose increase with high external salinity. These substances exhibit protective and ion-compensating effects and bear a high share of the total osmotic concentration for many species under ex- treme conditions (Greenway & Munns, 1980). Hence, our approach is to use freezing point osmometry, which seizes the concentration of osmotically active particles independent of their ionic or non-ionic character (Swee- ney & Beuchat, 1993). Plants change in osmotic potential parallel to that of the soil (Mitlöhner & Kopp, 2007). Failure of osmotic balance results in loss of turgidity, cell dehydration and, ultimately, death of cells (Gorham, 1995). Intracellular osmotic potential can decrease as a result of an accumulation of solutes or a decrease in cell water content (Turner, 1970) in which the former is considered as active (true) osmotic adjustment. Since the osmotic potential is highly variable among species, it can be used to compare the osmotic stress tolerance of species within their natural distributions (Abrams, 1988; Gebre et al., 1998) and reflect the concentration of dis- solved salts, sugars and organic acids in the cells (Mitlöhner & Koepp, 2007).
In principle, the water and osmotic potentials of leaves reaches their minimum at noon because of water loss due to high transpiration. In predawn, water and osmotic potentials reach their relative diurnal maximum owing to the re-hydration of the leaves (Kramer & Boyer, 1995). However, osmotic active substances accumulated within the cells of a plant are expected to be stored and reflect the solute content of the soil (Mitloehner & Koepp, 2007). The rationale for the measurements of water and osmotic potentials was the belief that more drought tolerant species tend to reach low water potentials more rapidly than less resistant species (Ladiges, 1975; Gebrekirstos et al., 2006), and plants with a higher solute accumulation in their leaves will tolerate higher salinity than those that do not succeed (Mitlöhner, 1998). The water and osmotic potential values can also be used to judge the suitability of a site for the introduction and promotion of both exotic and indigenous tree spe- cies (Mitlöhner, 1998; Gebrehiwot et al., 2005; Gebrekirstos et al., 2006).
We hypothesized that species with different growth forms and functional types differ in their drought toler- ance and osmotic adaptations. The main objectives of the study were, therefore, to: 1) carry out comparative analysis on the drought and osmotic stresses of co-occurring tree and shrub species under natural conditions; and 2) investigate if the economically important wild fruit tree, D. glabra, has a comparative ecophysiological ad- vantage in contrast to all co-occurring species. This knowledge is important in predicting their future (ecological) success in view of changes in site conditions and global climate.
1.1. Study Site
The study was carried out in Awash National Park (ANP), which is located within the Ethiopian Rift Valley at about 207 km East of Addis Ababa between 8˚45'N to 9˚15'N and 39˚45'E to 40˚5'E. The park covers ap- proximately 756 km2. The vegetation in the park is comprised of open grassland, shrubland, bushland and woodland savannah and gallery forest. The present study focused on the shrub and woodland savannah vegeta- tion. The exact location of the present study was in the central part of the park at an altitudinal range of 990 - 1130 m. The ANP was designated as Wildlife National Park since 1969 and is known for its high population of grazers, such as Oryx, Soemmering’s Gazelle and Swayne’s Hartebeest. However, the inhabitants around the ANP, belonging to the Kereyu, Ittu and Afar ethnic groups are predominantly pastoralists, mostly dependent upon the park for their livestock (camels, cattle and goats) (Gebrekirstos et al., 2006).
The local climate is semi-arid having a bimodal rainfall distribution with long and short rainy periods from July-September and February-April, respectively. Mean annual precipitation ranges between 400 and 700 mm. The distinct dry season with no or untimely rainfall and the lowest monthly day and night temperatures occur during October to January. The mean monthly temperatures range from 29˚C in November to 36.6˚C in June. The area is characterised by continuous sunshine coupled with high temperatures as well as low and erratic rainfall, which have resulted in high rates of evapo-transpiration. The soil types in the park are grouped accord- ing to the parent material. They are mixtures of regosols, andosols, solonchalks, histosols and fluvisols. The shrublands occur where welded tuff andosols, regosols, and solonchalks are common. The bushland and wood- land savannah are more commonly found on shallower alluvial and colluvial soils. Primary and secondary salin- ity problems are recognized throughout the Lower Awash Valley.
1.2. Study Species
Nine naturally co-occurring species of the dominant trees and shrub species growing under the same climatic conditions were selected for the present study. These were: a) deciduous trees, namely Acacia tortilis (Forssk.) Hayne, Acacia seyal Del. and Acacia nilotica (L.) Willd. ex
Del .
; b) deciduous shrubs, namely A. senegal (L.) Wild., Dichrostachys cinerea (L.) Wight & Arn., Acacia oerfota (Forssk.) Schweinf. and Acacia mellifera (Vahl) Benth.; and c) evergreen trees, namely Balanites aegyptiaca (L.) Del. and Dobera glabra (Forssk.) Poir.
The two evergreen species belong to the family Balanitaceae and Salvadoraceae, respectively, whereas all the Acacia species and Dichrostachys cinera are members of the Fabaceae family (Table 1). The plant water poten- tial values of the five species (A. tortilis, A. seyal, A. senegal, D. cinerea and B. aegyptiaca) were reported in our previous work (Gebrekirstos et al., 2006). We reported these results in this paper for comparison with their os- motic potential values and to get a broader picture of the ecosystem. We would like to note that field measure- ments and sample collections for all the study species, including those specie reported by Gebrekirstos et al. (2006), were carried out in December 2003. Despite the fact that the species co-occur, they differ in their niche preferences. Detailed description of the site characteristics, including history, flora and fauna, climate and soils of the site can be found in Abule (2002) and Gebrekirstos (2005).
1.3. Plant Water Potential
Plant water potential (Ψ) was measured using a pressure chamber (Scholander et al., 1965), which is a method
![]()
Table 1. Plant water potential measurements of the most common species in Awash National Park, Ethiopia. Means and standard errors are shown for each variable, within a column, and means followed by different letters were statistically dif- ferent at p < 0.05.
Note: N = number of trees examined.
widely used (Sellin, 1996). Extensive reviews on the pressure chamber measurements are available elsewhere (Tyree & Hammel, 1972; Ritchie & Hinckley, 1975; Boyer, 1995; Richter, 1997). The measurement was carried out during the late dry season in December 2003 before all the leaves were shed. Six to eight trees were ran- domly selected from each species, except for water potential measurements of A. mellifera for which only two trees were successfully measured due to difficulty in detection of sap droplets in the cutting ends. A study in a particular tree consisted of two measurements, at midday (Ψmd) (12:00-14:30) when water potential is expected to be in its daily minimum, and during predawn (Ψpd) (3:30-6:00) at the time of presumed highest water poten- tial. At the time of each measurement, freshly cut mature leaves from tree branches of south exposition situated at the same insertion height of 3 m (for shrubs) and 5 - 7 m (for trees) were used for each species.
1.4. Plant Osmotic Potential
Branches with leaves were collected, simultaneously, from the same individual plants (six to eight trees per spe- cies) on which Ψ measurements had taken place. The leaves were, then, detached and weighed in the fresh state under field conditions using a precision balance. Subsequently, leaves were dried using a kerosene stove, in or- der to avoid enzymatic changes, labelled and packed in the dry state for further laboratory processing at Burck- hardt-Institute of Tropical Silviculture and Forest Ecology, Göttingen, Germany.
Dry matter contents and determination of freezing points were carried out following standard cryoscopy pro- cedures described by Kreeb (1990). First, the leaves were dried in the oven at 105˚C for about 12 h. Then, the oven-dry weight was measured using a sensitive measuring balance. Relative leaf water content (LWC) related to the dry weight was calculated using the formula:
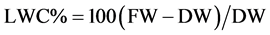
where FW is the fresh leaf weight in the field and DW is the kiln dry weight.
The dried leaves were grounded to a fine powder. One gram of each sample was set into an exact multiple of the original fresh mass diluted with 8 ml of distilled water. The suspension was soaked in water bath at 55˚C for 24 h to get the organic acids, sugars and salts dissolved completely. To separate leaf material from liquid, the samples were centrifuged for 25 minutes at 8000 rotations per minute using Labofuge III (Heraeus GMBH, Germany). The freezing points of the extracted solutions were determined using a cryoscopy (Knauer GMBH, Germany). A cryoscopy measures the concentration of total dissolved solutes by measuring the freezing-point depression of a solution, regardless of the nature of the solute (Mitlöhner & Köepp, 2007). Finally, the osmotic potential (Ψp) was recalculated from the freezing points of the samples to the previous water content according to the following equations (Kreeb, 1990):
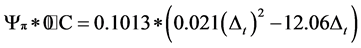
and to the actual air temperature at plant sampling time:
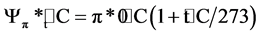 ,
,
where: ∆t = the depression at freezing point; p*0˚C = the osmotic potential at 0˚C ; pt˚C = the osmotic potential at t = ˚C.
The whole process of measurement, from field sample collection to laboratory measurements, resulted in er- rors less than ±0.2 MPa (Kreeb, 1990).
1.5. Data Analyses
The differences between respective Ψpd and Ψmd values were presented as diurnal range (ΔΨ), which indicates the range of daily relaxation. To visualize and evaluate the range of relaxation in the different species and to as- sess the impact of drought on plant water, the values of Ψmd and Ψpd values were used as the Y and X axes, re- spectively, and a 45˚ bisecting line was plotted on a graph, representing the boundary where midday and pre- dawn water potential have equal values. When the species fail to recover from the water stress during the day persistently, the values would approach the bisecting line, which could lead to leaf shedding and/or decline in growth of the tree (Mitlöhner, 1998; Gebrehiwot et al., 2005; Gebrekirstos et al., 2006). In other words, the ΔΨ value in the dry season shows the tolerance range of the species to water stress. The bisecting line is assumed to express site-specific limits of the species (permanent wilting point) in that particular site during dry season (Ge- brekirstos et al., 2006).
The same principle holds for Ψp as well. However, as osmotic change takes several weeks or days, daily re- laxation values will be used to assess passive (due to dehydration) from active (solute accumulation) osmotic adjustments. A wide diurnal change relative to leaf water content would indicate that the solutes were more concentrated due to leaf dehydration rather than to active osmotic adjustment.
Pair-wise mean comparison of midday and predawn values were carried out using paired t-test (Zar, 1999) in order to evaluate the position of the points on the graph in relation to the bisecting line. Differences among the species were determined using One-Way Analysis of Variance (ANOVA). Further, when significant variations in the ANOVA were revealed, means were compared using Tukey’s test (Fowler et al., 1998). STATISTICA for windows (Version 6.0) was used as a tool for the data analysis. Normal distribution of the data was tested using Chi-square goodness of fit. Unless stated otherwise, results are significant at p < 0.05.
2. Results
2.1. Response to Drought Stress
There was an overall significant difference among the species in midday, predawn and diurnal ranges during the dry season (Table 1). All species exhibited their lowest values during midday compared to predawn values. The species showed water potential values ranging from −0.83 MPa for A. senegal to −5.6 MPa for Dobera glabra (Figure 1). Of all the species, the evergreen D. glabra showed the lowest mean Ψmd (−4.8 ± 0.22 MPa) and Ψpd (−3.57 ± 0.20 MPa) values, followed by B. aegyptiaca (Ψmd value = −4.7 ± 0.19 MPa) and D. cinerea (Ψpd value = −2.94 ± 0.22 MPa). Acacia senegal showed the highest Ψmd and Ψpd values, although not significantly differ- ent from the values of A. oerfota, A. mellifera and A. seyal. Though the evergreen species exhibited lower Ψmd values than the deciduous species, there was no difference among the deciduous shrub and tree species.
All species exhibited substantial overnight water re-hydration as evidenced from the values, which are falling above the bisecting line (Figure 1), which, hypothetically, represents the permanent wilting points. Furthermore, the paired t-test between midday and predawn values confirmed significant night recovery from the midday wa- ter stress for all the species. There were significant differences in ΔΨ among the species. Balanites aegyptiaca showed the highest ΔΨ (2.17 ± 0.22 MPa) followed by A. tortilis (1.6 ± 0.22 MPa). The lowest ΔΨ were found in A. oerfota (0.68 ± 0.25 MPa) and A. senegal (0.72 ± 0.23 MPa).
![]()
Figure 1. Midday and predawn values of plant water potential measurements (MPa) of the most common species in Awash National Park, Ethiopia.
2.2. Response to Osmotic Stress
There was a significant difference in the Ψp values among the species (Table 2). The standard error values ob- tained from all species were very low, indicating the reliability of the measurements. All species exhibited their lowest Ψp values during midday. Dobera glabra revealed the lowest Ψpmd (−3.04 ± 0.08 MPa) and Ψppd (−2.92 ± 0.08 MPa) values followed by A. oerfota with Ψpmd, and Ψppd values of −2.62 ± 0.08 MPa and −2.56 ± 0.08 MPa, respectively. Acacia mellifera, A. seyal, A. senegal, and B. aegyptiaca showed no significant differences in their Ψpmd values whereas A. senegal showed a significantly higher Ψppd value of −1.60 ± 0.07 MPa. In contrast to the Ψ values, Ψp values did not show distinct variations among the different functional groups and growth forms.
There were no significant differences in diurnal ranges (ΔΨp) among the species (Table 2). In contrast to the water potential values (Figure 1), values of Ψp of all species fell along the bisecting line (Figure 2). Moreover, the paired t-test between Ψpmd and Ψppd values showed no significant difference for all the species, which might indicate active osmotic adjustments. Although LWC and Ψp were not correlated across species, the two species showing lowest Ψp tended to have high LWC (Table 2). Though both evergreen species showed higher LWC, there was no clear difference among the different functional groups.
3. Discussion
The plant water and osmotic potential values ranged from high to low. The categorization of the species, into high and low, according to water potential values was not consistent with the osmotic potential ranking. In fact, these two parameters are independent and a similar category of the species was not expected. From our results, it is evident that different species exhibited different degrees of adaptation to the conditions of water and osmotic stress.
Decline in Ψmd compared with Ψpd in all species can be attributed to reduced availability of water, and similar findings were reported from other areas (Sellin, 1998; Mitlöhner, 1998; Gebrehiwot, 2003; Gebrekirstos et al., 2006). The Ψmd value is expected to indicate the least favourable condition to the plant since it integrates and re- flects all environmental influences on the internal water balance of the plant (Gebrekirstos et al., 2006). Gener- ally, the evergreen trees attained lower leaf water potentials compared to the deciduous tree and shrub species, which concurs with findings of Sobrado (1986). However, significant difference between the evergreen species in Ψpd, i.e. between D. glabra and B. aegyptiaca were also found. This could be due to different rooting depths or soil solute concentrations.
The narrow ΔΨ in the dry season, which indicate lack of ability to re-hydrate during the night in the dry sea- son, have been recognised as indicators of water deficiency (Mitlöhner, 1998; Gebrehiwot et al., 2005; Gebre- kirstos et al., 2006). Despite the brown appearance of leaves and almost leaflessness of most of the deciduous species during the time of measurement, all the species exhibited a significant relaxation. However, three days
![]()
Table 2. Plant osmotic potential measurements of the most common species in Awash National Park, Ethiopia. Means and standard errors are shown for each variable, within a column, and means followed by different letters were statistically dif- ferent at p < 0.05.
Note: N = number of trees examined.
before the measurement there was anomalous rain of about 3.1 mm, which might have changed the soil moisture conditions.
Similar to Ψ, all species exhibited their lowest Ψp values during midday, when soil water availability was low and, hence, solute concentration was presumed to be higher. In fact, the low Ψp values could be due to solute concentration as a result of cell dehydration or solute accumulation in response to stress (Morgan, 1984). Since build-up of osmotic adjustment usually requires several days or weeks, the diurnal fluctuation may be due to changes in relative water content (Abrams, 1988). In agreement, the decrease in Ψp during midday is consistent to the decrease in LWC at midday (Table 2). However, the diurnal Ψp change in our study is minimal, which might indicate active osmotic adjustment. Although LWC and Ψp were not correlated across species, the two species having the lowest Ψp exhibited high LWC. This seems to be due to their higher solute concentration in a given volume of tissue as reflected by their lower potentials.
Ranking of species according to Ψ and Ψp under field conditions reveals some interesting differences among species. Unlike Ψp, there was no clear difference in Ψp between functional types and growth forms of species. Nevertheless, the degree of adjustment was clearly distinct among the species, and not all plants fit closely into one or the other category. Our results demonstrated the importance of different adaptive characteristics at dif- ferent habitats and its implications to their ecological success in different niches. Kreeb et al. (1995) reported that large differences in soil parameters were recorded over very short distances, and these differences were strongly correlated with the pattern of vegetation. Low Ψp values have been associated with drought tolerance (Morgan, 1984; Gebre et al., 1997) and indicators of soil salinity (Mitlöhner & Koepp, 2007). Mechanisms of salt tolerance take place at molecular, cellular and whole plant levels (Munns, 2002). The cellular response of salt tolerant organisms to salinity stress includes the synthesis and accumulation of compatible solutes (Apse & Blumwald, 2002).
Outstandingly, the evergreen D. glabra, which inhabits calcarious and salty soils, showed the lowest Ψ and Ψp values among the study species. This implies that D. glabra is highly drought and osmotic stress tolerant. On the other hand, since the early responses to water and salt stress are essentially identical, one may wonder if the lower osmotic value (compared to the co-occurring species) is due to water stress or accumulation of solutes to adapt to the calcareous and salty soils. We believe that the later is the case. Mitlöhner & Köepp (2007) carried
out green house measurements under fully saturated situation in order to understand the possible influence of soil osmotic concentrations on plant osmotic concentrations. Their results indicated that the tree and soil osmotic potential approached a 1:1 relationship. Hence, based on their field and laboratory measurements, they
![]()
Figure 2. Midday and predawn values of plant osmotic potential mea- surements (MPa) of the most common species in Awash National Park, Ethiopia.
concluded that plant osmotic potential can serve as “bio-indicator” of soil osmotic conditions. Breckle (2002) takes it as a rule that the plant osmotic potential exceeds the soil osmotic potential by −0.5 - 1 MPa. The lowest Ψ and Ψp values of D. glabra compared with the co-occurring species further imply that the species has a com- parative advantage to thrive in drought and salinity prone areas. This finding is in agreement with similar results from other studies (Mitlöhner, 1998; Gebre et al., 1997). Under circumstances of severe stress, species with low osmotic values will be at an advantage. Consistent to our conclusions based on physiological traits, Tsegaye et al. (2007) characterised D. glabra as drought tolerant species based on seedling survival in a field experiment. In their studies on the recruitment of D. glabra in different land use systems, Tsegaye et al. (2010) recorded higher electrical conductivity in plots that had higher number of adult individuals of D. glabra. Hence, the ecophysi- ological characteristics mentioned above explain why this important species is widely distributed in the ex- tremely dry and saline areas in the Afar National Regional State (ANRS), north eastern Ethiopia.
The highest predawn water potential values of A. senegal, A. oerfota, A. mellifera and A. seyal indicate easy access to water. The lack of differences in predawn water potential among the study species suggests that they have similar effective rooting depth (Hinckley et al., 1978). For instance, based on plant water potential mea- surements (Gebrekirstos et al., 2006) as well as climate growth relationships and stable carbon isotopes (Gebre- kirstos et al., 2008; Gebrekirstos et al., 2011) A. senegal and A. seyal have been ranked as drought avoiders and B. aegyptiaca, A. tortilis and D. cinerea as drought tolerant species. When looking at the Ψp values, however, D. cinerea showed the highest Ψp values among all the studied species, followed by A. tortilis and A. senegal. In contrast to their high plant water potential ranking, A. oerfota showed low Ψp and A. seyal and A. mellifera be- longed to intermediate Ψp ranking. As evidenced by its highest Ψp values, A. tortilis prefers rocky scraps to allu- vial soils and avoids seasonally water logged alkaline soils (not osmotic stress tolerant), while low Ψp values in- dicate its drought tolerance. Acacia seyal prefers seasonal waterlogged areas where high solute concentration is expected. The low Ψp value of D. cinerea indicates its competitive ability in areas where water availability is a limiting factor, while its high Ψp values might indicate its relative low osmotic stress tolerance.
Mitlöhner (1998) recorded –17.3 MPa as the most extreme Ψp value for trees in Paraguay Chaco. In our study, –2.9 MPa predawn, daily minimum value of –3.28 MPa, and daily fluctuations of about 0.01 - 0.18 MPa were recorded. In a similar study in Northern Ethiopia (annual rainfall = 656 mm), Gebrehiwot (2003) reported minimum osmotic potential values of –1.23 MPa with significant diurnal fluctuation ranges of 0.15 - 0.42 MPa, which were measured in the late dry season. The difference in Ψp values are interesting, but not surprising, given the fact that our study area is one of the drought and salinity prone areas in Ethiopia. Drought, salinity, and ex- treme temperatures are often interconnected (Wang et al., 2003) and manifested primarily as osmotic stress (Serrano et al., 1998). In addition, excess evapo-transpiration draws water from deeper soil layers and causes many soluble minerals to accumulate in the upper soil horizon (Abrol et al., 1988). For instance, around Awash River, which is located close to our study area, farms have been abandoned due to salinity caused by inappropriate irrigation practices coupled with high evapo-transpiration. This area is currently invaded by Prosopis juliflora (Sw.) DC., an exotic woody species, which thrives well on saline soils (Shiferaw et al., 2004).
Lack of leaching in arid regions also causes the accumulation of salts released by weathering in the process of soil formation, leading to dryland salinization (Gunn & Richardson, 1979). Overgrazing is known to decrease vegetation cover and as the most pervasive causes of land degradation. Fire increases the soil concentration of mineral elements, reduces moisture (Wright & Bailey, 1982) and elevates alluvium salinity (Bush & Smith, 1993). Fire occurrence in savannahs is a well-established fact. Hence, the frequent drought occurrences coupled with overgrazing and past and present frequent fires might have contributed to the high solute concentration in our study area. It is worth noting that recently, the grasslands and the A. senegal (L.) Willd. shrubland are en- croached by A. oerfota and A. mellifera (Abule, 2002). It, therefore, seems that the expansion of A. oerfota and A. mellifera may be related to their tolerance of osmotic stress, which may imply a trend of soil salinization.
4. Conclusion
This study examined the plant water relations of D. glabra and co-occurring species with the aim of examining their drought and osmotic stress tolerance and comparative advantages. The Ψ values of species of different growth forms and functional types and diurnal differences revealed significant differences among the species. The differences among the species suggest that the species respond differently to water and osmotic stress, and that they employ different strategies to offset the deleterious effects of drought and osmotic stress. The ever- green species showed lower Ψ compared to deciduous species but no differences were observed between the different growth forms (trees and shrubs). With regard to Ψp, no significant difference was observed between the functional groups and growth forms of species. The differences in water relations obtained among the species reflected their niche preferences and have further implications on their distribution and tolerance range in case of climate change-induced drought and changes of site conditions.
We can conclude that in case of progressive drought, the species with lower water potentials will be at an ad- vantage, while in areas where soil salinity and drought prevail species having lower osmotic and water potentials will thrive. Our results have further implication in our efforts of domestication to tackle food insecurity in drought prone areas. The ANRS, where our study area is located, is known for high food insecurity and recur- rent drought periods. Therefore, in our efforts to promote agroforestry and tree planting to tackle food insecurity in dry lands, we strongly recommend domesticating the valuable indigenous fruit tree, D. glabra, as it is a highly drought and salinity tolerant species. Also, we recommend future studies to further investigate the detailed pro- cesses involved in adaptation of the species to saline soils (e.g. whether it involves the regulation of uptake and/ or compartmentalization of salt) and the corresponding impact on growth and productivity. The division of plant responses into categories or strategies can be a valuable aid to understanding the ecological consequences for a species, e.g. potential for carbon acquisition and growth during drought, long-term plant survival and distribu- tion, monitor the present site condition and predict the direction of future changes.
Acknowledgements
The study was financed by the German Academic Exchange Service (DAAD), which is gratefully acknowl- edged. We thank Wondo Genet College of Forestry in Debub University, and Prof. Masresha Fetene for logisti- cal support. We extend our thanks to the staff members of Awash National Park for their kind assistance and lo- gistical support during the fieldwork.
NOTES
*Corresponding author.