1. Introduction
Many of coumarin derivatives are biologically active [1] [2] . A great volume of studies have attempted to inhibit bacterial growth using naturally occurring coumarins, such as herniarin, umbelliferone and scopoletin on the an- tifungal activity of umbelliferone, scopoletin and coumarin itself [3] [4] . Some coumarin derivatives, including novobiocin and analogues, have been demonstrated to be very active as antibiotics [5] -[7] . Among synthetic de- rivatives, several antibacterial 3-acyl and 3-carba-moyl-4-hydroxycoumarins [8] -[12] have been described.
Bis-coumarins are generally prepared by condensing carbonyl compounds with 4-hydroxycoumarin in organic solvents [13] [14] , which applied a large number of hazardous and toxic solvents related to catalysts. Several methods have been recently reported for such synthesis which includes use of different catalysts such as mole- cular iodine [15] , MnCl2 [16] , strong tertiary amine base (DBU) [17] , POCl3 [18] , diethyl aluminum chloride (Et2AlCl3) [19] , LiClO4 [20] , SO3H functionalized, ionic liquids [21] , SDS [22] , TBAB [23] , Zn(Proline)2 [24] , [bmim] [BF4] [25] , sulfamic acid [26] , RuCl3∙nH2O [27] , SiO2Cl [28] , SiO2-OSO3H NPs [29] , Sulfated titania [30] refluxing in ethanol or acetic acid [31] , thermal solvent-free microwave, ultrasound condition [32] [33] etc.
With the growing public concern about the environment, several synthesis methods using 4-hydroxycoumarin have been recently proposed for preparing biscoumarins in aqueous media. Despite the efficacy and eco-friend- liness of these methods, they use catalysts including TEBA [34] and I2 [35] and have long reaction times. Thus, it is still required to propose efficient and novel methods based on green methodology.
p-Dodecylbenzenesulfonic acid (DBSA) is a Brønsted acidsurfactant-combined catalyst, which is composed of an acidic group and a hydrophobic moiety. It could be explained that the inside of emulsion droplets com- posed of substrate and DBSA is hydropho-bic enough to exclude water molecules. Therefore, surfactant-cata- lyzed organic reactions in water have turned to be one of the most challenging research issues. The behavior of DBSA as a catalyst has been studied in Mannich type reactions, Biginelli reaction, synthesis of bis (indol-3-yl) alkanes, tetrahydrobenzo [b] pyrans, dihydropyrano [c] chromens, xanthenes derivatives and esterification of various carboxylic acids and alcohols [36] -[42] . In the present work, these results were reported for the synthesis of 3,3-Arylidene bis(4-hydroxycoumarin) derivatives by DBSA in environmentally benign conditions and under microwave irradiation.
2. Result and Discussion
In this paper, efficient method was tried to be proposed for the condensation of aldehydes with 4-hydroxycou- marin, which led to the corresponding 3,3-arylidene bis(4hydroxycoumarin) in the presence of DBSA as a ho- mogeneous catalyst in two methods (A, B) (Scheme 1). DBSA was used as a source of H+ to catalyze this reac- tion and found to be a good catalyst for the preparation of 3,3-arylidene bis(4-hydroxycoumarin).
Initially, the systematic evaluation of different solvents for the model reaction of 3-nitro benzaldehyde and 4-hydroxycoumarin in the presence of DBSA in water at reflux was focused on. Attempts were made to study and optimize the reaction conditions in order to show that performing the reaction in H2O with low yield while using the amounts of EtOH in the media produced satisfactory results (Table 1, entry 6). These results revealed that the highest yield was obtained with the water/ethanol (1:1) solvent system (Table 1, entry 6).
Since DBSA was emerged as a suitable catalyst for the reaction in 1:1 ethanol/water media, then efforts were made to optimize the catalyst load for the condensation reaction, leading to the rapid formation of 3,3-arylidene bis(4-hydroxycoumarin). The present optimization studies revealed that the yield smoothly increased with the catalyst load up to 25 mol% and the use of larger amounts of the catalyst did not improve the yields, while its decreasing amount decreased the yields. The negligible amount of the product was formed in the absence of catalyst.
To find the specific effect of microwave irradiation on the reaction, these reactions were carried out under the same conditions in a microwave oven (Table 1, entry 13) and it was observed that, while the reaction time considerably decreased, the yields of the product slightly increased. Thus, MW conditions had a beneficial effect on this reaction. Afterward, concentration was within the scope of this reaction with the variety of aldehydes (Scheme 1) in order to check the viability of this protocol in obtaining a library of 3,3-arylidene bis(4-hydrox- ycoumarin) derivatives in two methods (Table 2).
As can be seen in Table 2, a range of dicoumarols was synthesized using different aldehydes and 4-hydrox- ycoumarin under the standardized reaction. The results are summarized in Table 2. Regardless of the nature of the substitution (electron donating and electron withdrawing) of the aromatic aldehydes, the products were ob- tained in good to excellent yields (entries 1 - 17). Similar results were also obtained in the microwave condition
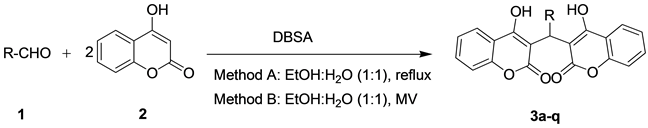
Scheme 1. Synthesis of 3,3-arylidene bis(4-hydroxycoumarin).
![]()
Table 1. Optimization of reaction condition on the yield of 3,3-arylidene bis(4-hydroxycoumarin).
aIsolated yield.
![]()
Table 2. Synthesis of 3,3-arylidene bis(4-hydroxycoumarin) by condensation of aldehydes and 4-hydroxycoumarin using DBSA (25 mol%) as catalyst.
Method A: EtOH:H2O (1:1), reflux; Method B: EtOH:H2O (1:1), MV.
(Method B). All the reactions were completed within 45 - 150 min and 4.5 - 15 min in Methods A and B, re- spectively. In these reactions, there was no need for the column purification of the products. The obtained solid products were just filtered off from the reaction mixture, dissolved in hot ethanol, refiltered to separate any con- taminated catalyst with the product and finally recrystallized from the filtrate to obtain pure dicoumarols.
According to the proposed mechanism, the formation of 3,3-arylidene bis(4-hydroxycoumarin) could be ra- tionalized from the Knoevenagel condensation of aromatic aldehydes with 4-hydroxycoumarin in the presence of DBSA and followed by Michele addition of the second 4-hydroxycoumarin (Scheme 2).
To show the advantage of the present work in comparison with the reported results in the literature, the results of DBSA with reflux in ethanol or acetic acid, iodine, DBU, SDS, TBAB, TiO2/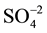 and SiO2/Cl were com- pared in terms of the synthesis of biscoumarin derivatives. As shown in Table 3, DBSA can act as an effective catalyst with respect to reaction time, yields and the obtained products.
and SiO2/Cl were com- pared in terms of the synthesis of biscoumarin derivatives. As shown in Table 3, DBSA can act as an effective catalyst with respect to reaction time, yields and the obtained products.
In conclusion, DBSA was demonstrated a new efficient catalyst for the synthesis of dicoumarols, prepared via the condensation reaction of aromatic aldehyde and 4-hydroxycoumarin using DBSA as a homogeneous catalyst under aqueous media and microwave conditions. These conditions had advantages such as shorter reaction time, simpler work-up, inexpensive and non-toxic catalysis, environmental benignity and excellent yields.

Scheme 2. Proposed mechanism.
![]()
Table 3. Comparison of our results with some of those reported in the literature for the reaction of 4-nitro benzaldehyde, malononitrile and 4-hydroxycoumarin.
3. Experimental
3.1. Instruments and Characterization
IR spectra were recorded on a Perkin-Elmer FT-IR 240-C spectrophotometer (KBr). 1H-NMR and 13C-NMR spectra were run on Bruker spectrometers at 400 MHz for 1H-NMR and 100 MHz for 13C-NMR. The melting points were determined using an Electrotermal 9100 apparatus. The reactions were monitored by thin layer chromatography and the products were identified either fully or in the comparison of melting points and spec- troscopic data with the previously reported ones.
3.2. General Procedure for the Synthesis of 3,3-Arylidene Bis(4-Hydroxy-2Hchromen-2-Ones) Derivatives (Method A)
A mixture of 4-hydroxycoumarin (2 mmol, 0.324 g), substituted benzaldehydes (1 mmol, 0.106 g), and DBSA (0.25 mmol, 0.326 g) was stirred at reflux in 5 ml ethanol-water mixture (1:1). The progress of the reaction was monitored by TLC. After the reaction completion and upon its cooling, the solid material was precipitated from the solution. The precipitates were filtered off, washed with water, and were recrystalized from EtOH to obtain pure 3,3-arylidene bis(4-hydroxy-2Hchromen-2-ones) derivatives as yellow-white solids (60% - 90% yields).
3.3. General Procedure (Method B)
A mixture of 4-hydroxycoumarin (2 mmol, 0.324 g), substituted benzaldehydes (1 mmol, 0.106 g), and DBSA (0.25 mmol, 0.326 g) in 5 ml ethanol-water mixture (1:1) was inserted in a microwave oven (Samsung, Model KE300R) at 450 W for an appropriate period of time (Table 1, Method B). The reaction was followed by TLC. After the reaction completion, its mass was cooled down to 25˚C. The solid residue were filtered off, washed with water, and were recrystalized from EtOH (68% - 93% yields).
3.4. The Spectral Data of Unreported Compound 3q
4-hydroxy-3-(((4-hydroxy-2-oxo-2H-chromen-3-yl)(2-(prop-2-ynyloxy)naphthalen-3-yl)methyl)-2H-chromen-2-
one (3q): Mp = 168˚C - 172˚C. IR (KBr, cm−1): 3450, 3271, 3074, 2148, 1661. 1H NMR (400 MHz, CDCl3): δ = 1.58 (s, 1H, CH), 2.34 (t, 1H, J = 2.4 Hz, ≡CH), 4.56 (dd, J = 9.6, 2.4 Hz, CH2-O), 6.44 (m, 2H, H-arom), 7.37 - 7.43 (m, 6H, H-arom), 7.64 - 7.69 (m, 4H, H-arom), 8.06 (s, 2H, H-arom), 10.90 (s, 1H, OH), 11.75 (s, 1H, OH). 13C NMR (100 MHz, CDCl3) δ: 34.3, 56.9, 116.6, 116.7, 118.0, 118.1, 119.2, 124.3, 124.4, 124.5, 124.7, 124.8, 124.9, 130.9, 132.6, 132.9, 130.1, 132.6, 132.9, 133.1, 133.8, 135.2, 152.1, 152.2.
NOTES
*Corresponding author.