1. Introduction
Corrosion of a metal is said to be due to chemical or electrochemical reactions when it comes in contact with matters present in its environment. However, corrosion can be controlled by suitably modifying the environment which stifles the anodic or cathodic reaction or both, and this is achieved by the use of inhibitors [1] .
Mild steel has been extensively used under different conditions in chemical and allied industries in handling alkaline, acid and salt solution. One way of protecting mild steel from corrosion is the use of organic inhibitors [2] . The known hazardous effects of most synthetic corrosion inhibitors are the motivation for the use of some natural products. The exploration of natural products as the corrosion inhibitor is becoming the subject of extensive investigation due to low cost and eco-friendliness of these products and their biodegradability. This method is fast replacing the synthetic and expensive hazardous organic inhibitors [3] . The yield of these compounds as well as their corrosion inhibition abilities varies widely depending on the part of the plant and its location [4] . The extracts from the leaves, seeds heartwood, bark, root and fruit of plant have been reported to inhibit metallic corrosion in acidic media. Researchers have reported the use of local plant such as Italian Vernonia amygdalina as the corrosion inhibitor for aluminium [5] [6] . In line with this, Allamanda blanchetii has been reported as the effective inhibitor for mild steel in citric acid and sulphuric acid [7] .
Veawab, Totinwachuthikul & Chakma [8] noted that ferrous materials, especially mild steel, are largely used in acidic media in most industries including oil/gas exploration and ancillary activities. They stated that during such activities, inhibited sulphuric acid is widely used in pickling, descaling and stimulation of oil wells in order to increase oil and gas flow [8] .
Inhibitors currently employed in corrosion works are varied and some have been found to be hazardous to health and the environment at large. Thus efforts are now directed towards formulation of modern environmentally safe inhibitors in which plants extracts have become important because they are eco-friendly; are economically cheap; are readily available; and are renewable sources of effective corrosion inhibitors [9] .
Therefore, the present investigation is directed at the evaluation of extracts from Amaranthus cordatus as inhibitors for fighting the corrosion of mild steel in H2SO4 and NaCl media employing weight loss technique.
2. Experimental Techniques
2.1. Materials/Experiment
The materials and equipment used for the work include 10 mm diameter mild steel rods sourced from a local steel stockist in Enugu, Nigeria, beakers, digital weighing balance, tetraoxosulphate (VI) acid, leaves of Amaranthus cordatus, acetone, nylon strings, emery cloth, distilled water, hacksaw, vernier calliper, measuring cylinder, and volumetric flask.
2.2. Materials Preparation
The mild steel rods were cut to sizes, each averaging 94.5 cm2 in surface area. They were thoroughly brushed with emery cloth to reveal the metal surface. Thereafter, they were washed with distilled water and swabbed in acetone. They were weighed after drying to note the initial weight. The tetraoxosulphate (VI) acid and Sodium Chloride were prepared to 0.5 M and 1.0 M concentration using standard procedures. The Amaranthus cordatus leaves were washed with cold tap water, dried under room temperature; after which they were subjected to soxhlet extraction process in ethanol for about 80 hours to obtain the extract.
2.3. Experimentation
The mild steel coupons were tied with nylon strings and then suspended in beakers containing the acid and the base as well as the hydrolysed and acidified extracts. Each beaker contained 5 coupons and the entire set up were allowed to stand for 30 days. After 6 days a coupon was withdrawn from each beaker, rinsed in distilled water and swabbed in acetone. Thereafter they were reweighed for weight loss determination, corrosion rate and inhibition efficiency calculation using the formulas:
 ; and
; and

where cpr, Δw, ρ, A and t are corrosion penetration rate (mm/yr), weight loss (g), density, total surface area and exposure time (hours) respectively; while 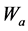 and
and  are the values of weight losses in absence and presence of inhibitor respectively, and IE% depicts Inhibition Efficiency in percentages. The pH value of the Amaranthus cordatus extract was evaluated and noted.
are the values of weight losses in absence and presence of inhibitor respectively, and IE% depicts Inhibition Efficiency in percentages. The pH value of the Amaranthus cordatus extract was evaluated and noted.
3. Results
Table 1 and Table 2 show the inhibition efficiency of the plant extract in controlling corrosion of mild steel at different extract volumes; while Figures 1-4 show the effect of Amaranthus cordatus on corrosion penetration rate of mild steel at room temperature.
4. Discussion
A look at Figures 1-4 shows that the corrosion rates obtained were typical of those of passivating metals. This is usually associated with an initial steep rise in corrosion rate, then peaking at a maximum and subsequently de-
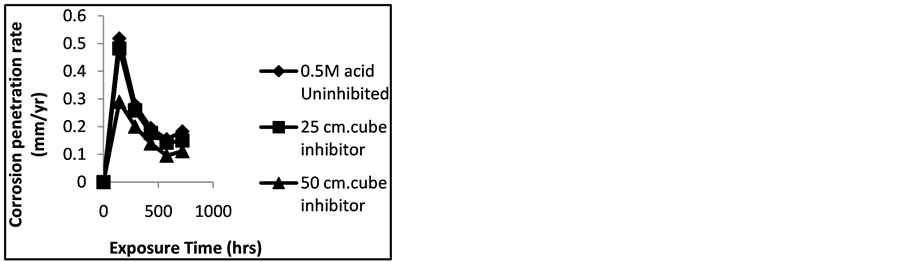
Figure 1. Effect of plant extract on corrosion penetration rate of mild steel in 0.5 M H2SO4 at room temperature.

Table 1. Inhibition efficiency of mild steel in H2SO4 in presence and absence of Amaranthus cordatus.

Table 2. Inhibition efficiency of mild steel in NaCl in presence and absence of Amaranthus cordatus.

Figure 2. Effect of plant extract on corrosion penetration rate of mild steel in 1.0 M H2SO4 at room temperature.

Figure 3. Effect of plant extract on corrosion penetration rate of mild steel in 0.5 M NaCl at room temperature.

Figure 4. Effect of plant extract on corrosion penetration rate of mild steel in 1.0 M NaCl at room temperature.
creasing as exposure time increased. It can also be seen that the rate of corrosion of mild steel decreased with increase in volumetric values of the extracts and the highest corrosion recorded was without inhibitor. The metal surface on interaction with the corrosion medium normally reacts swiftly with the medium forming an oxide that coats the entire surface and acts as a barrier, thereby preventing further reactions [10] .
4.1. Molar Concentration of Acids
The corrosion rates during six days the experiment lasted were all below the acceptable minimum (put at 0.5 mm/yr for most metals) in the entire media (although in varying proportions). The lowest corrosion rate of 0.10 mm/yr was achieved at 50 cm3 of the plant extract in 0.5 M H2SO4 while the lowest corrosion rate of 0.0004 mm/yr was achieved at 50 cm3 of the extract in 0.5 M NaCl. This implies that the molarity of the acid or base had a significant effect on the corrosion rate, agreeing with the works of previous researchers [11] [12] . However, the values obtained for the two molar concentrations of the acid and base showed that at higher molarities, the corrosion rates were higher comparatively, and although most of the corrosion penetration rate values fell well below the permissible limits, the trend indicated a decrease in corrosion rate as exposure time increased.
4.2. Volumetric Concentration of the Extract
It has been established that corrosion decreases as the concentration of plant extracts increases [12] [13] . In a similar fashion, the corrosion penetration rates decreased as the volumetric values of the extract increased. This can be attributed to the increase in the amount of plant extract adsorbed on the metal surface, thus reducing the available sites for either acid or base attack [14] . Hence, it shows that Amaranthus cordatus is a good inhibitor. The inhibition efficiencies however varied with the concentration of the acid or base such that corrosion rate values were higher at higher acid or base concentrations.
4.3. Inhibition Efficiency of Amaranthus cordatus
From the tables the maximum inhibition efficiency of the plant extract at the end of the 30 days of the experiment was 47.80% in 0.5 M H2SO4 and 99.51% in 0.5 M NaCl. This is because the attack of the acids main reacting agent (hydrogen ion) is severe as it is being evolved without replacement thus leaving the reaction system insufficient of the major component of the reaction. This reduces the rate of the oxidation of the mild steel, thus increases the efficiency of the plant extract to inhibit its corrosion while NaCl has less attack on the reaction system, hence inhibition efficiency increases as the extract volumes increase. The rate of inhibition is appreciable at lower concentration than higher concentration of the acid or base. The maximum inhibition efficiency recorded at the end of 30 days was due to the adsorption of constituents of the plant extract on the mild steel passivating it from further corrosion. This is also in line with the established fact that mechanism of adsorption of the plant extract on the surface of mild steel is a physical adsorption [15] .
4.4. Comparison of Amaranthus cordatus Inhibitory Action
It was observed that the inhibitory character of the plant extract is more pronounced in Figure 3 and Figure 4 than in Figure 1 and Figure 2. This shows that inhibitor strength decreased more in H2SO4 than in NaCl. The gap between uninhibited and inhibited samples shows that the plant extract is inhibiting the corrosion of mild steel. The plant extract has been proven in this work to have good inhibitory behaviour in the field of corrosion. Hence, Amaranthus cordatus in both H2SO4 and NaCl show good inhibitory character. So inhibition efficiency of Amaranthus cordatus increased tremendously in NaCl when compared to H2SO4 at room temperature.
5. Conclusion
Based on the results obtained and the foregoing discussion, an inference is drawn to the fact that Amaranthus cordatus inhibits the corrosion of mild steel in H2SO4 and NaCl solution to an appreciable extent. Hence, is a good inhibitor which could cause passivation, since its pH value of 8.1 falls within the region in which passivation occurs in the pourbaix diagram [16] . Again, the increase in inhibition efficiency with increase in volumetric values of the plant extract and decrease in concentration of the acid at room temperature show that the plant extract is physically adsorbed on the mild steel coupon. Therefore, the extract of Amaranthus cordatus can be considered as a source of non-toxic, environmentally friendly and effectively green corrosion inhibitors in acid and base media for mild steel.
Acknowledgements
The Department of Industrial Chemistry, Ebonyi State University, Abakaliki, Nigeria is acknowledged for permitting the authors to use their laboratory facilities for this work.