1. Introduction
Measurements of carbon dioxide (CO2)-evolution from soils are important in evaluating biomass and activity of soil microorganisms, as well as decomposition of soil organic matter. Several methods have been developed for the determination of soil CO2-evolution, both under laboratory and field conditions [1] -[6] proposed a conductimetric method for measuring soil CO2-evolution under field and laboratory conditions. According to [5] , the absorption of CO2 by an electrolyte, such as potassium hydroxide (KOH), produces a change in the conductance of the solution. [7] improved this principle to a fully computerized sampling system (Respicond VI) allowing continuous measurements both in short- and long-term (less than one day up to several weeks) soil incubation experiments. However, the application of this method was limited to incubations at constant temperatures [8] -[10] . This is in contrast to natural field conditions with diurnal and weather determined fluctuations of soil temperatures. The objective of the present investigation was to develop a method and a calibration procedure for the Respicond VI allowing measurements of soil CO2-evolution under changing temperatures. The proposed temperature correction procedure will completely eliminate the influence of the temperature on the conductance during long-time incubation.
2. Materials and Methods
2.1. Principles of the Respicond
The main principle of the Respicond respirometer has been described previously [7] . Briefly, it consists of water baths with inserted hermetically closed vessels for soil incubation. Each vessel includes a smaller open vessel with KOH solution and platinum electrodes inserted into the solution. Each set of electrodes is connected to a digital volt-/amperemeter and a source of constant current (Figure 1). The CO2 released from the soil sample is absorbed by the KOH solution. The amount of absorbed CO2 is determined by measuring changes in conductivity of the KOH solution. Data from each vessel are collected in hourly increments and analyzed using the software supplied with the Respicond VI. The chemical reaction of the absorption of CO2 by the KOH solution is
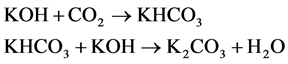
The concentration of ions in solution decreases linearly in relation to the amount of absorbed CO2. The amount of accumulated CO2 during incubation N(CO2) can be calculated using the following equation (reformulated from [7] ):
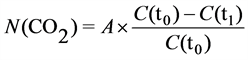 (1)
(1)
where C(t0) was the conductance at the beginning of the incubation (time t0) before any absorption of CO2 by KOH, C(t1) was the conductance at time t1 and A was an empirically determined constant expressing the theoretical maximum amount of CO2 absorbed and it can be calculated as A = N(CO2) if C(t1) = 0. This calculation assumes that the temperature of the solution is constant during the experiment. The constant A is proportional to the volume and concentration of the solution. The default value of the constant A used by the software of the Respicond VI is 219 mg CO2 for 10 ml of 0.6 M KOH solution, corresponding to 36.5 mg CO2 ml−1 1 M KOH. Another published value for constant A is 9.94 mg CO2 for 5 ml of 0.05 M KOH solution [5] , corresponding to 39.8 mg CO2 ml−1 1 M KOH. No further calibrations for other concentrations of KOH are available, although, a reliable detection of low CO2 evolution rates (<100 µg CO2 h−1) is possible only with lower concentrations of KOH solution [7] . To meet the objective of this study, it was necessary to re-determine the value of the constant A for suitable concentrations of KOH, its dependence on the temperature and finally to develop a method to perform measurements under variable temperatures to allow for variations in experimental conditions or induced temperature treatments.
2.2. Calibration
The determination of the constant A can be done using two different approaches [5] [6] :
1. Production of a defined amount of CO2 in a closed vessel following the reaction
![]()
Figure 1. Principle design of a Respicond VI incubation vessel and the measuring unit.
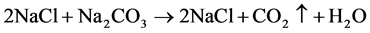 The emitted CO2 is absorbed by the KOH solution, resulting in a changing conductance of the KOH solution.
The emitted CO2 is absorbed by the KOH solution, resulting in a changing conductance of the KOH solution.
2. Replacing the KOH solution by mixtures with different ratios of KOH and potassium carbonate (K2CO3). According to Reaction 1, this imitates the absorption of defined amounts of CO2.
To apply the first calibration method, 10 ml 1.0 M hydrochloric acid (HCl) were added to empty vessels. After closing the vessels, sodium carbonate (Na2CO3) solution was injected through a septum into the HCl. Twelve injections were made, each of which with 1 ml 0.2 M Na2CO3 producing 8.8 mg CO2. After each injection, the conductance of the KOH solution (10 ml, originally 0.5 M ) was measured as soon as it stabilized (approx. 30 minutes). The whole experiment was repeated five times at 20˚C.
The constant A was calculated from the linear relationship between the CO2 evolved and expected to be absorbed by KOH (N(CO2)), and the measured conductance after injection C(tinj):
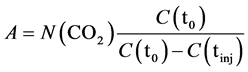 (2)
(2)
where C(t0) and C(tinj) were the conductance of KOH solution before and after injection, respectively.
For the second calibration method, it is assumed that the absorption of one mole of CO2 converts two moles of KOH to one mole of K2CO3 and one mole of H2O (see reactions above). The absorption of CO2 could be simulated by replacing KOH with CO2-equivalent amounts of K2CO3 solution. We mixed 0.5 M , 0.1 M and 0.05 M KOH with 0.25 M , 0.05 M and 0.025 M K2CO3, respectively, each of which at 6 different ratios of KOH to K2CO3: 0/10, 2/8, 3/7, 5/5, 7/3, 9/1 at 20˚C. Each combination of concentrations and ratios was replicated six to seven times.
To investigate the influence of temperature on the calibration of the constant A, the experiment with 0.5 M KOH and 0.25 M K2CO3 was conducted again at six different temperatures (5˚C, 10˚C, 12˚C, 15˚C, 20˚C and 25˚C) with three experimental replicates for each temperature. According to Equation 2, constant A was calculated from the linear relationship between the equivalent amount of CO2(Eq(CO2)), and the corresponding measured conductance :
:
 (3)
(3)
where CKOH was the conductivity of the original KOH solution.
To detect a possible temperature dependency of the constant A, measurements at 6 temperatures from 5˚C to 25˚C were fitted to the following linear function:
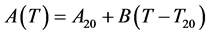 (4)
(4)
where T20 = 20˚C, T is the actual temperature, A20 is the value of the constant A (mg CO2) at 20˚C and B is the regression coefficient indicating temperature dependency.
Linear regressions and corresponding coefficients of determination were calculated. An analysis of variance was performed to identify significant differences between calibrations with different molarity of the KOH solution.
2.3. Correction for Changing Incubation Temperatures
The conductance of the KOH solution is positively correlated with temperature. Equation (1) assumes that the temperature is constant during incubation. Thus, the conductance must be recalculated if the temperature changes. During incubation at a constant temperature (ideal condition), the conductance in vessels without soil does not change and any change in conductance measured in these control vessels is only related to changes in temperature during incubation. Fluctuations of conductance in vessels without soil can, thus, be used to calculate a correction factor (Fcorr(t0,t1)) for changes in temperature between times t0 and t1:
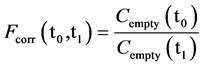 (5)
(5)
where Cempty(t0) and Cempty(t1) are the conductances measured in the vessel without soil at time t0 and t1, respectively. By assuming equal temperatures in vessels with and without soil, the temperature corrected conductance Ccorr(t1) in the vessels with soil can be calculated for t1 using the following equation:
 (6)
(6)
To verify this method, we performed the following experiment. Soils were placed into plastic vessels with hermetic lids and were pre-incubated at 20˚C for 24 hours before starting the experiment. The temperature scheme of the incubation experiment in the Respicond VI water bath was ten days at 20˚C, eight days at 25˚C and another four days at 20˚C. Two vessels without soil were used to calculate the correction factor Fcorr(t0,t1). Then, this correction factor was used to correct the conductance in vessels with soil. Five soil samples (0 - 60 cm) from two experimental sites in the state of Baden Württemberg (Southwest Germany) were used: one sample from a Calcaric Cambisol (silty loam, 0.7% organic C, pH(CaCl2) 6.4) and four samples from a Rendzic Leptosol (clay loam, 1.9% organic C, pH(CaCl2) 7.9). Soil samples were sieved ( 2 mm ) and adjusted to water content of about 50% maximum water holding capacity. Three replicates were performed for each soil sample resulting in a total of 15 vessels.
3. Results and Discussion
A nearly perfect linear relationship between the relative change of conductance and (equivalent) amounts of absorbed CO2 was found (Figure 2(a) and Figure 2(b)). Calculated values of constant A are shown in Table 1. For the 0.5 M KOH solution, both calibration methods gave nearly identical results (A = 173.3 ± 1.4 and 175.8 ± 1.6 mg CO2). Since these results were statistically independent, we suggest using the average value A = 174.5 mg CO2. This corresponds to 34.9 mg CO2 ml−11 M KOH and is, thus, lower than the default value used by the Respicond VI software corresponding to 36.5 mg CO2 ml−11 M KOH (see above). In calibration procedure 2, constants A for 0.1 and 0.05 M KOH corresponded to values of 34.0 and 33.4 mg CO2 ml−1 1 M KOH, respectively. These values did not differ significantly from the value 33.4mg CO2 ml−1 1 M KOH determined for 0.5 M KOH solution, indicating that the constant A must not be calibrated separately for KOH solutions differing in concentrations, at least within the range we investigated.
The temperature dependence of constant A is shown in Figure 3. Fitted values of A20 and Bin Equation (4) were 173.4 ± 1.0 and 0.26 ± 0.14, respectively. Since B was not significantly different from 0 (p > 0.05), it can be concluded that constant A does not depend on the temperature. The fitted value of A20 was again very close to the above-suggested mean value of constant A.
The method of temperature correction was validated in an incubation experiment with soils under two temperature conditions. The temperature correction factor, Fcorr(20˚C, 25˚C) was found to be 1.083 ± 0.001. The proposed temperature correction procedure completely eliminated the influence of the temperature on the conductance (Figure 4).
![]()
![]()
Figure 2. Relative change of conductance in the KOH solution relative to initial values (see Equation (2)) depending on (a) the amount of CO2 released after injection of Na2CO3 into HCl and assumed to be absorbed by the KOH solution and (b) the amount of absorbed CO2 equivalent to the K2CO3 replacing KOH in the solution. Data were fitted to linear functions forced through the origin. Error bars show standard errors ((a) n = 5, (b) n = 6) and are partly hidden by the symbols.
![]()
Figure 3. Relationship between the temperature in the water bath as the difference to the standard temperature of 20˚C (DT20 = 20˚C-actual temperature) in the water bath and the calculated constant A, where 0.5 M potassium hydroxide (KOH) was replaced by0.25 M potassium carbonate (K2CO3) to imitate carbon dioxide absorption. Error bars (standard deviations, n = 3) are partly hidden by the data symbols.
![]()
Figure 4. Conductance of 0.5 M KOH solution during incubation at 20˚C/ 25˚C/20˚C with and without calculated temperature correction. Average of 15 vessels.
![]()
Table 1. Mean values (± standard deviations) of A calibrated using two different methods: Either production of a defined amount of CO2 by injection of Na2CO3 into HCl assumed to be absorbed by the KOH solution (Calibration 1) or replacing the KOH solution with mixtures of KOH and K2CO3 in different ratios imitating absorption of definite amounts of CO2. Values in brackets show A converted to a KOH concentration of 0.5 M, assuming a linear relation to concentration.
4. Conclusion
We suggest using 174.5 mg CO2 as constant A for the conversion of conductance to evolved CO2 adsorbed in 10 ml 0.5 M KOH during soil incubation in the Respicond VI. This value of A corresponds to 34.9 mg CO2 ml−1 1 M KOH. Within the ranges we investigated, constant A does neither depend on the incubation temperature nor on the concentrations of the KOH solutions. To eliminate the influence of changing temperatures in the Respicond VI during incubation on the conductance of the KOH solution, a correction factor calculated from changes in conductance of the KOH solution in incubation vessels without soil should be used. We recommend using the temperature correction procedure for incubation experiments with temperature instability of 1˚C or more and for the measurement of the respiration rate even with stable temperature conditions. Our findings may also be applicable to earlier and later versions of the Respicond respirometer.
Acknowledgements
We thank Anders Nordgren for cooperation and valuable comments, and we acknowledge the kind suggestions by Pavel Krokovny. This research was part of sub-project P3 within PAK 346, “Structure and Functions of Agricultural Landscapes under Global Climate Change-Processes and Projections on a Regional Scale” and FOR 1695 “Agricultural Landscapes under Global Climate Change-Processes and Feedbacks on a Regional Scale” with funding provided by the German Research Foundation (DFG). Natalya Smirnova was a collaborator via the “International Academic Mobility Network with Russia” (IAMONET-RU) as a part of the “Erasmus Mundus External Cooperation Window” funded by the European Commission.
NOTES
*Corresponding author.