Statistical Studies of the Physicochemical Analytic Results of a Series of Synthetic Calcium Hydroxyapatite Containing Carbonate and Sodium ()
1. Introduction
Carbonated calcium hydroxyapatite containing sodium and/or potassium, magnesium etc. is the most important mineral compound in human dental, enamel and bone [1] [2] . We can also found this type of apatite in sedimen- tary phosphate apatite [3] . Consequently, these materials have necessitated studies of the effects of ions, such as 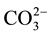 Na+, Mg2+, on the physicochemical properties and especially the mode of
Na+, Mg2+, on the physicochemical properties and especially the mode of 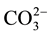 Na+ incorporation in apatite structure. But, the complexity of biological and sedimentary phosphates apatite has returned these studies very difficultly.
Na+ incorporation in apatite structure. But, the complexity of biological and sedimentary phosphates apatite has returned these studies very difficultly.
For this reason, multiple techniques have been used to prepare these products, with wet chemical methods and solid-state reactions [4] -[14] . Carbonate incorporation in synthetic carbonated calcium hydroxyapatites “CO3 HAp” has been classified as either type A or type B depending on the mode of 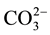 substitution:
substitution: 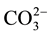 for
for  type A substitution and
type A substitution and 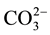 for
for 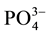 type B substitution [10] [11] .
type B substitution [10] [11] .
In the precipitation of “CO3HAps”, different products are obtained if the calcium solution is added to the phosphate plus carbonate solution “direct” rather than vice versa “inverse” apatite [6] [8] [12] -[14] . All these products are B-Apatite, but it seems that the maximum possible carbonate content was greater in the “direct” apatite compared with “inverse” apatite [13] . Na-free B-type “CO3HAps” has been prepared with a maximum 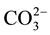 content between 10 wt% at 14 wt%. In the presence of ions such as (Na+, K+ or Mg2+), we have a coupled substitution. With Na+ for Ca2+, B-type apatite “Na-CO3HAps” contains up to 22 wt%
content between 10 wt% at 14 wt%. In the presence of ions such as (Na+, K+ or Mg2+), we have a coupled substitution. With Na+ for Ca2+, B-type apatite “Na-CO3HAps” contains up to 22 wt% 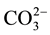 [14] .
[14] .
Based on some studies, such as EPR and IR spectroscopy, X-ray and neutron diffraction, chemical analyses, infrared and Raman spectroscopy, the crystallite size, chemical composition and physicochemical nature of synthetic apatite have been determinated. But there have been multiple studies, speculations and controversy about the mechanism(s) by which 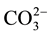 and alkali metal(s) are incorporated in the apatite lattice [15] -[20] . According to De Maeyer et al., the fundamental substitution mechanisms for the incorporation of Na+ and
and alkali metal(s) are incorporated in the apatite lattice [15] -[20] . According to De Maeyer et al., the fundamental substitution mechanisms for the incorporation of Na+ and 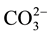 in hydroxyl apatite “HAp” can be described using six basic processes [21] .
in hydroxyl apatite “HAp” can be described using six basic processes [21] .
1) 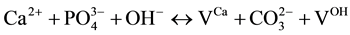
2) 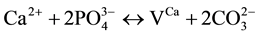
3) 
4) ![]()
5) ![]()
6) ![]()
where VX is the vacancy on a regular apatite lattice site occupied by X.
Moreover, these authors propose a coupling of different fundamental mechanisms in a fixed proportion, lead- ing to the definition of apparently new mechanisms. The composition of B-type of Na-CO3HAps is determined by the occurrence of one or more of these fundamental mechanisms. Thus, these authors suggest that the stoi- chiometry would be given by:
![]()
where a, b, c, d and e are the contribution per unit cell of basic substitutions (I to V) resulting in the fundamental substitution mechanisms for incorporation of Na+ and ![]() in the crystal lattice of hydroxyapatite “HAp”.
in the crystal lattice of hydroxyapatite “HAp”.
The aim of the present study is to present a statistical method which allows us to determine the relationship between the obtained values and experimental conditions and to estimate the change in one variable from the given increase or decrease in another. The analysis of variance (ANOVA) allows us to test the mathematical model. Finally, the statistical method allows us to find the fundamental substitution mechanism(s) for incorpora- tion of Na+ and![]() . So, the stoichiometry of the solid can be described as a function of the contributions of each mechanism to the composition of the unit cell.
. So, the stoichiometry of the solid can be described as a function of the contributions of each mechanism to the composition of the unit cell.
2. Experimental Procedure
2.1. Preparation of Na-CO3HAps
The preparation method is described in detail elsewhere [12] [13] , In brief, The precipitated Na-CO3HAps were prepared by dripping calcium solution 0.03 M (Ca(NO3)2×4H2O) into a phosphate solution 0.008 M of (Na2HPO4×12H2O) which contains also different concentrations of (Na2CO3) such as the molar ratio R:
![]() . So that, the concentrations of
. So that, the concentrations of ![]() and [Na+] were varied in the hydrolysis solution:
and [Na+] were varied in the hydrolysis solution:![]() ,
,![]() . Then, the concentration ratio
. Then, the concentration ratio ![]()
After hydrolysis which took 3 h, the precipitates were filtered, washed abundantly with hot distilled water (95˚C), dried for 12 h at 70˚C and then heated at 400˚C in air for 24 h in order to eliminate inter- and intra- crystalline water.
2.2. Characterization
2.2.1. Infrared Spectroscopy and X-Ray Diffraction
Na-CO3HAps samples are characterized by IR absorption spectroscopy and X-ray diffraction. In infra-Red, we use pellets of absorption. They are prepared using the usual KBr disk technique. It consists in mixing 1 mg of powder of a sample of Na-CO3HAp with 300 mg KBr then pressed at 6 psi. The pellets so prepared were then scanned on a Shimadzu IR spectrometer in the range (4000 - 400) cm−1.
Powder X-ray diffraction analysis for the Na-CO3HAps samples were carried out using an X-ray diffractome- ter MRD with a generator (40 kV and 40 mA). After indexation of the full pattern, cell parameters were refined using the program “WINCELL”
2.2.2. Chemical Analysis and Density Measurements
The dehydrated samples were subjected to a chemical analysis. The phosphorus content of precipitates is deter- mined by colorimetry after complexation with vanado-molybdate [22] . The sodium and calcium content are ob- tained by atomic absorption spectroscopy and the carbonate content was determined by coulometry method (re- lease of CO2 by dissolution in acid). The hydroxide content % OH was calculated on the basis of electro neutral- ity. Density of the solids was measured by a flotation method [13] .
3. Results
3.1. Physical Analysis
The infra-Red spectrums presented in Figure 1 show that they are typical of apatite containing B-type carbonate [13] . The assignment of absorption bands was made according to the studies [12] [13] . IR analyses of the sam- ples show the effect of increasing ![]() and Na+ on the spectral properties of apatites. However, increasing
and Na+ on the spectral properties of apatites. However, increasing ![]() and Na+ contents caused increasing of the intensities of the
and Na+ contents caused increasing of the intensities of the ![]() absorption bands at 1420 - 1460 cm‒1 (n3 C‒O) and 872 cm‒1 (n2 C‒O) and decreasing in the resolution of
absorption bands at 1420 - 1460 cm‒1 (n3 C‒O) and 872 cm‒1 (n2 C‒O) and decreasing in the resolution of ![]() (n3 P‒OH) absorption band at 1032 cm−1, The shoulder observed at 740 cm‒1 is attributed to the nL mode of OH‒ close neighbors to Na+ ions [13] .
(n3 P‒OH) absorption band at 1032 cm−1, The shoulder observed at 740 cm‒1 is attributed to the nL mode of OH‒ close neighbors to Na+ ions [13] .
![]()
Figure 1. Absorption Spectra of Na-CO3Haps obtained from solutions with: (A) R = 5, (B) 15 AND (C) 25.
In Figure 2 we presented the X-ray diffraction patterns. The peaks are relatively sharp and well resolved and can all attributed to the hexagonal crystal form of hydroxyapatite, but some shifts of peaks position can be ob- served, reflecting a change in unit cell dimensions due to incorporation of ![]() and Na+. This allows us to consider our samples as pure well-crystallized phases of apatite type. The results of dimensions “a” and “c” and the volume of the hexagonal apatitic unit cell obtained from X-Ray analysis as well as the densities of the com- pounds allow determining the molar weight (Mw). The results are grouped together in Table 1.
and Na+. This allows us to consider our samples as pure well-crystallized phases of apatite type. The results of dimensions “a” and “c” and the volume of the hexagonal apatitic unit cell obtained from X-Ray analysis as well as the densities of the com- pounds allow determining the molar weight (Mw). The results are grouped together in Table 1.
3.2. Chemical Results
The compositions of samples in Weight % determined by chemical analysis are displayed in Table 2. The results of quantitative analysis of phosphorus, calcium and sodium were determined with standards deviations 0.17; 0.03 and 0.09 respectively and the amount of CO3 was determined on relative uncertainty 2%.
The results of the chemical and physical analysis (Table 1 and Table 2) allowed us to calculate the number of each ion X per unit cell, nx according to:
![]() , (1)
, (1)
where M (r×Vcell×N) is the molar mass, Vcell is the unit cell volume, Mx is the atomic or ionic mass of X and N Avogadro’s constant. The amount of OH ions was calculated taking into account the electroneutrality of each compound. Table 3 gathers the calculation results.
4. Discussion
As in the case of the reference [23] , the last column of Table 3 shows that the sum of the number of phosphate and carbonate per unit cell ![]() is approximately equals to 6 with experimental error as well as the XRD and IR spectra clearly demonstrate that the samples “Na-CO3HAps” of this study are pure B-type carbonated apatites, this indicates that
is approximately equals to 6 with experimental error as well as the XRD and IR spectra clearly demonstrate that the samples “Na-CO3HAps” of this study are pure B-type carbonated apatites, this indicates that ![]() substitutes for
substitutes for ![]() on a 1:1 basis which agrees with the fundamental substitution mechanisms for B-type
on a 1:1 basis which agrees with the fundamental substitution mechanisms for B-type ![]() [21] [23] -[25] . On the basis of the observation, the real number of each ion per unit cell can be obtained using the following equation:
[21] [23] -[25] . On the basis of the observation, the real number of each ion per unit cell can be obtained using the following equation:
![]()
Figure 2. XRD patterns of synthetic apatites obtained from solutions with: (A) R = 5, (B) 15, (C)25.
![]()
Table 1. Values of lattice parameters “a”, “c” and volume molar; density and weight (Mw) of the prepared samples.
![]()
Table 2. Chemical composition (weight %) and total mass balance S% of Na- CO3HAps prepared from solutions with![]() .
.
![]() (2)
(2)
Similarly, we have calculated the number of vacancies on Ca2+ and OH− lattice sites respectively:
![]() (3)
(3)
and
![]() (4)
(4)
The results of these calculations are summarized in Table 4. The errors in Table 4 were estimated by means of error propagation theory.
![]()
Table 3. Unit cell composition of Na-CO3AHps calculated on the basis of the chemical composition and by means of Equa- tion (1).
![]()
Table 4. Unit cell compositions of Na-CO3Aaps calculated on the basis of the chemical composition and by means of fol- lowing Equation (2).
Comparing our experiment data with the data available in the literature [24] -[26] , we can found that the presents results are in agreement with those of reference [25] , though, this series was prepared by hydrolysis of monetite in solution with varied ![]() and alkali metal Na+ concentration.
and alkali metal Na+ concentration.
So, it may be said in the present study, that the fundamental substitution mechanisms (I, III and IV) could ac- count for the incorporation of ![]() and Na+ in the HAp lattice. If a, c and d are the contributions of mechan- isms I, III and IV respectively, thus,
and Na+ in the HAp lattice. If a, c and d are the contributions of mechan- isms I, III and IV respectively, thus,
![]() (5)
(5)
![]() (6)
(6)
and
![]() (7)
(7)
and the generic formula has the following expression:
![]()
5. Statistical Studies
The objective of this study is to construct a mathematical model which allows us to estimate the change in Y the dependent variable from a given increase or decrease in X the independent variable and to determine which me- chanism(s) is related with experimental conditions.
The mathematical model is expressed as:
![]() (8)
(8)
where ei is the random variable drawn from N(0, s2), b0 and bj are the estimated regression coefficients. The model assumes that their deviation ε from the line is normally distributed with means 0 and constant variances s2.
Least square method [26] allows calculating the regression and correlation coefficients, the variance of the b parameters and to test the null hypothesis H0: bj = 0 and their significances level. The analysis of variance for the linear regression or the F test allows us to be confident that at least one of X-variable contributes to the regression. The theoretical basis of these calculations is given in references [12] [26] .
5.1. Multiple Linear Regression of Y = c/a Crystallographic Parameters Ratio on ![]() and X2 = % Na+ the % Weightions in the Hexagonal Apatite Lattice
and X2 = % Na+ the % Weightions in the Hexagonal Apatite Lattice
In attempts to disentangle and to measure the effects of the insertion of ![]() and Na+ ions on the hexagonal apatite lattice dimensions, we have constructed a multiple linear regression on two X-variables where,
and Na+ ions on the hexagonal apatite lattice dimensions, we have constructed a multiple linear regression on two X-variables where, ![]() and X2 = %Na+ (data Table 2) and Y is the estimate ratio of the hexagonal lattice dimensions (da- ta Table 1). The results of the calculation values of the different correlations coefficients, the estimated values of b0, b1 and b2, the variances and t-test of b1 and b2 are summarized in Table 5(a). The analysis of the variance for the linear regression or the F-test is given in Table 5(b).
and X2 = %Na+ (data Table 2) and Y is the estimate ratio of the hexagonal lattice dimensions (da- ta Table 1). The results of the calculation values of the different correlations coefficients, the estimated values of b0, b1 and b2, the variances and t-test of b1 and b2 are summarized in Table 5(a). The analysis of the variance for the linear regression or the F-test is given in Table 5(b).
The multiple linear regression (Table 5(a)) indicates that the ratio of the hexagonal lattice dimensions of Na-CO3Aps “c/a” vary linearly with the carbonate and sodium content according to:
![]() (9)
(9)
The analysis of variance (Table 5(b)/ANOVA) shows further that F-test = 100.5 is higher than criterion F(5%; 2.9) = 4.26. This allowed us to rejet H0: b1 = b2 = 0 and affirm that at least one of the predictors is linearly asso- ciated to the response.
T-test of of the estimated regression coefficients b1 and b2 has shown that b2 and b1 are significant at 70% level.
5.2. Multiple Linear Regression of ![]() the Molar Ratio in Na-CO3HAps Solid on the Concentration of Na+: X1,i and the Concentration of
the Molar Ratio in Na-CO3HAps Solid on the Concentration of Na+: X1,i and the Concentration of![]() : X2,i in Aqueous Solution
: X2,i in Aqueous Solution
To understand the influence of the experimental conditions on the composition of these apatites (data Table 4), we conducted a multiple linear regression [22] on two X-variables where, X1 and X2 are the Na+ and ![]() ions
ions
(a) ![]() (b)
(b) ![]()
Table 5. calculation for fitting a multiple linear regression analysis of the estimated Y = c/a the ratio of the lattice parameters of Na-CO3HApson carbonate and sodium contents X1 = wt% CO3, X2 = wt% Na. (a) Calculation of regression, correlation coefficients and variances, (b) F-test: Analysis of Variance.
concentrations in solution and Y is the estimates molar ratios of ![]() in the solids. The values of the different correlations coefficients, the estimated values of b0, b1 and b2, their variances and the F-test or the test of the variance are summarized in Table 6 and Table 7.
in the solids. The values of the different correlations coefficients, the estimated values of b0, b1 and b2, their variances and the F-test or the test of the variance are summarized in Table 6 and Table 7.
The multiple linear regression (Table 6(a)) indicates that the molar CO3/P ratio of the Na-CO3HAps vary linearly with the concentrations of carbonate and sodium in solution according to:
![]() (10)
(10)
The result of the F-test (Table 6(b)) F = 635.5 and the individual t-test of the b’s Table 6 b for b1, t = −7.82. For b2, t = 8.26 confirm that the molar CO3/P ratio of Na-CO3HAps solid is significantly depends on both con- centrations of carbonate ![]() and sodium [Na+] = X2 in aqueous solution. However, constantly we have
and sodium [Na+] = X2 in aqueous solution. However, constantly we have![]() , thus the expression in brackets
, thus the expression in brackets![]() , hardly varied, thus,
, hardly varied, thus,
CO3/P molar ratio is constant. This shows that for ![]() concentration ratio in solution constant the
concentration ratio in solution constant the
increase on molar ![]() in solid is equal to the decrease on molar
in solid is equal to the decrease on molar![]() . Thus, the molar ratio in the solid is not significantly influenced by the increase of Na+ and
. Thus, the molar ratio in the solid is not significantly influenced by the increase of Na+ and ![]() ions in solution. This result is in a good agree- ment with the results in literature [24] .
ions in solution. This result is in a good agree- ment with the results in literature [24] .
The multiple linear regression (Table 7(a)) indicates that the molar Na/P ratio of Na-CO3HAps vary linearly with the carbonate content according to:
![]() . (11)
. (11)
An analysis of variance (Table 7(b)/ANOVA), further shows that F-test = 43.5 is higher than criterion F(1%, 2.10) = 7.56, this allowed us to rejet H0: b1 = b2 = 0 and affirm that at least one of the predictors is linearly as- sociated to the response. T-test of the regression coefficients b1 and b2 has shown for b1, t = −0.45 signifiant at 30% level. For b2, t = 1.67 signifiant at 80% level.
5.3. Multiple Linear Regression of the Estimated Molar Content of Calcium ![]() on
on ![]() and
and ![]() the Molar Contents of Sodium and Carbonate in the Solid
the Molar Contents of Sodium and Carbonate in the Solid
The examination of Table 4 showed too that nCa molar content of the solid decreases when ![]() and nNa incor- porate increase. To determine the relationship between these three variables given in Table 4, a multiple linear regression analysis was undertaken. The results are summarized in Table 8.
and nNa incor- porate increase. To determine the relationship between these three variables given in Table 4, a multiple linear regression analysis was undertaken. The results are summarized in Table 8.
From Table 8, the multiple linear regression analysis shows that the relationship between these quantities is given by equation:
(a) ![]() (b)
(b) ![]()
Table 7. Calculation for fitting a multiple linear regression analysis of the estimated Y = nNa/nP molar ratio in Na-CO3HAps solid on X1 = [Na+] and ![]() the concentrations in solution. (a) Calculation of regression and correlation coefficients and variances, (b) Values of F-test: Analysis of Variance.
the concentrations in solution. (a) Calculation of regression and correlation coefficients and variances, (b) Values of F-test: Analysis of Variance.
![]() (12)
(12)
From the intercept of the following equation it can seen that, within experimental error, a carbonate-free apa- tite ![]() contains 10 Ca2+ ions per unit cell. The expression in brackets
contains 10 Ca2+ ions per unit cell. The expression in brackets ![]() shows that the number of Ca2+ ions in the unit cell decreases with increasing of Na+ and
shows that the number of Ca2+ ions in the unit cell decreases with increasing of Na+ and ![]() numbers for about one Ca2+ ion.
numbers for about one Ca2+ ion.
The analysis of variance (Table 8(b)), further shows that F-test = 379.4 is higher than criterion F(5%, 2.9) = 4.26, this allowed us to rejet H0: b1 = b2 = 0 and affirm that at least one of the predictors is linearly associated to the response. T-tests of the regression coefficients b1 and b2 show that the value of: b1 is signifiant at 80% level and for b2 is signifiant at 90% level.
5.4. Multiple Linear Regression of Y = a, c or d the Contributions of Mechanisms I, III and IV on X1 = [Na+] and ![]() the Concentrations in Solution
the Concentrations in Solution
For determine which mechanism(s) is related with experimental conditions, we have undertaken a statistical analysis [26] of the values of a, c and d the contribution of the mechanisms I, III and IV (Table 9) calculated according to the following Equations (5)-(7) as a function of Na+ and ![]() concentrations in aqueous solution. Results of these calculations are summarized in Tables 10-12.
concentrations in aqueous solution. Results of these calculations are summarized in Tables 10-12.
![]()
Table 9. Values of (a, c and d) the estimated contribution of the mechanism I, II and IV.
From Table 10, the multiple linear regression analysis shows that the relationship between these quantities is given by equation:
![]() (12)
(12)
An analysis of variance (Table 10(b)/ANOVA), further shows that F-test = 25.36 is higher than criterion F (5%, 2.9) = 4.26, this allowed us to rejet: is linearly H0: b1 = b2 = 0 and affirm that at least one of the predictors associated to the response. The individuals t-test of the regression show that for b1, t = 0.79 signifiant at 60% level and for b2, t = −0.70 signifiant at 50% level.
From Table 11, the multiple linear regression analysis shows that the relationship between these quantities is given by equation:
![]() (13)
(13)
An analysis of variance (Table 11(b)/ANOVA), further shows that F-test = 163.55 is higher than criterion F(5%, 2.9) = 4.26, this allowed us to rejet H0: b1 = b2 = 0 and affirm that at least one of the predictors is linearly associated to the response. The individual t-tests on the regression coefficients b1 and b2 and, Hence, for b1, t = 10, for b2, t = −9.8 t(b1) and t(b2) are signifiants at P > 99.9% level.
From Table 12, the multiple linear regression analysis shows that the relationship between these quantities is given by equation:
![]() (14)
(14)
An analysis of variance (Table 12(b)/ANOVA), further shows that F-test = 0.10 is lower than criterion F(5%, 2.9) = 4.26, this test affirm that mechanism IV is unrelated to experiment conditions. The result of the present study provide that mechanisms I and III are the main in incorporation of Na+ and ![]() in HAp.
in HAp.
5.5. Multiple Linear Regression Analysis for the Determination of Formula of Unit Cell
The determination of unit cell has been realized by a multiple linear regression between the variables Y = nCa and X1 = a, X2 = c, X3 = d. Least square allows calculating the regression and correlation coefficients. The sample regression (prediction equation) is:
![]() , where
, where ![]() are the estimated regression coefficients. These have been calculated from the values of correlation coefficients, variance and covariance according to the method of Scherrer [27] . The results of calculations are given in Table 13. To testing the utility of the model, we conduct the F-test according to:
are the estimated regression coefficients. These have been calculated from the values of correlation coefficients, variance and covariance according to the method of Scherrer [27] . The results of calculations are given in Table 13. To testing the utility of the model, we conduct the F-test according to:
![]() , (15)
, (15)
where n is sample size, m is number of parameters and ![]() is degree of freedom.
is degree of freedom.
From Table 13, the multiple linear regression analysis shows that the relationship between these quantities is given by equation:
![]() (16)
(16)
(a) ![]() (b)
(b) ![]()
Table 12. Calculation for fitting a multiple linear regression of the estimated Y = d = nNa ? c the contribution of the mechanism IV on the concentration of Na+: X1,i and the concentration of![]() : X2,i in aqueous solution, (a) Calculation of regression and correlation coefficients and variances, (b) Values of F-test: Analysis of Variance.
: X2,i in aqueous solution, (a) Calculation of regression and correlation coefficients and variances, (b) Values of F-test: Analysis of Variance.
![]()
Table 13. Calculation for fitting a multiple linear regression of the estimated Y = nCa =Y in Na-CO3HAps on the contribution of mechanism I a: X1i, contribution of mechanism III c: X2i contribution of mechanism IV d: X3i.
An analysis of variance (ANOVA), further shows that F-test = 2018.9 is higher than criterion F(5%, 2.9) = 4.26, this allowed us to rejet H0: b1 = b2 = b3 = 0 and affirm that at least one of the predictors is linearly asso- ciated to the response.
for testing the regression coefficients b1, b2 and b3 and discovering with variable(s) is related to estimate Y = nCa, we conduct on the one hand individual t-tests on the b’s, Hence, for b1, t = −5.28, for b2, t = −28.3 and for b3, t = −1.2 t(b1) and t(b2) are signifiants at P > 99.9% level. Thus, the molar nCa of Na-CO3Aps solid is signifi- cantly depends on both a and c contributions of mechanisms I and III. The general formula can be written as fol- lows:
![]()
Also, structural study for two samples obtained under comparable conditions [28] has been investigated ex- tensively by physicochemical analysis and by Rietveld method refinements. The results of unit cell content cal- culated from the occupations of the atomic sites and the data of chemical composition ![]() may be represented by the following ideal substitution scheme and corresponding solid solution :
may be represented by the following ideal substitution scheme and corresponding solid solution :
![]()
(The sum of mechanism II and III)
![]() , with x =1.5 (I) and 2.4 (II).
, with x =1.5 (I) and 2.4 (II).
These results show that we cannot consider unique and well defined substitution mechanisms resulting in apa- tites, especially for homogeneous precipitation methods in aqueous solutions, because the lack of control of the reaction parameters as well as incomplete analyses of the solids could result in erroneous interpretations of the substitution mechanism.
On the other hand El Feki et al. [28] confirm that no vacancies of OH− are observed by Rietveld refinements. But, small fraction of vacancies is undetectable by this method and was ignored in the structure refinements.
6. Conclusion
The different statistical analyses present in this review mainly focused on original and new approaches of the knowledge of the substitutions mechanisms. However, in biological calcifications, part of lattice ions of Hap are substituted to considerable extent ions. Consequently, these substitutions have an important influence on several processes (the growth, the dissolution, the mineralization and the demineralization processes. In order to derive the fundamental thermodynamic properties of the solid which determine the course of these processes, the stoi- chiometry of the apatite and especially of the mechanisms by which ![]() and Na+ are incorporated in the lat- tice must be known.
and Na+ are incorporated in the lat- tice must be known.