One-Pot Production of Substituted Anthraquinones via the Diene Synthesis in the Presence of Mo-V-P Heteropoly Acid Solutions ()
1. Introduction
9,10-Anthraquinone (АQ) and its derivatives are the well known organic products [1] -[3] . They are used in production of dyes [2] , hydrogen peroxide [3] , medicals [3] . They are also applied as wood delignification catalysts [3] . Diene synthesis is known as a process for anthraquinones production. In case of substituted anthraquinones this process is based on reaction between 1,4-naphthoquinone (NQ) and substituted 1,3-butadienes [1] [3] . It is also possible to use naphthoquinones with the same substitutes in positions 6, 7 and (or) 5, 8. Primary addition products (adducts) are obtained in organic solvents (AQ synthesis is preformed under 1,3-butadiene pressure of 0.3 - 2 МПа or in the presence of organic acids for pressure reduction). Diene synthesis adducts may be then oxidized to AQ in acidic medium by strong oxidants (CuCl2, H2O2 or NaClO3 [4] ) or by air oxygen in alkaline medium [5] . In the latter case the process is rather slow. Thus 24 hours are required to transform the adduct of NQ reaction with 2,3-dimethylbutadiene into the corresponding AQ in 5% alcohol solution of potassium hydroxide at room temperature under atmospheric pressure [6] .
We have already developed [8] -[11] a one-pot process for the synthesis of non substituted 9,10-anthraquinone from NQ and 1,3-butadiene in the presence of high vanadium modified heteropoly acids solutions (HPA) of non-Keggin brutto composition H15P4Mo18V7O89 (HPA-7) and H17P3Mo16V10O89 (HPA-10), allowing a 70% AQ yield, main substance content attaining 97%. This process is performed in the butadiene atmosphere at 80˚C, molar ratio HPA:NQ being 2:1. Mixing with water organic solvents (acetone and 1,4-dioxane) is used for the purpose, their molar ratio to the aqueous HPA solution being 1:2. Being simultaneously strong Brøensted acids and oxidants, HPA solutions act as bi-functional catalysts, i.e. acid catalysts for the diene synthesis and oxidation catalysts for the adducts synthesis.
In the present study, we have used the above described method to obtain the substituted 9,10-anthraquinones in the presence of Мо-V-P HPA solutions.
2. Experimental
In all studies, we used 1,4-naphthoquinones, isoprene, 1-methyland 2,3-dimethylbutadienes Alfa Aesar. Chloroprene (2-chlorobutadiene) was prepared according to known method [12] by dehydrochlorination of 3,4- dichlorobutene-1 with 20% NaOH solution.
0.2 M solution HPA-10 (brutto-composition H17P3Mo16V10O89) was prepared according to method described in [13] . The 51V and 31P NMR spectra of the HPA-10 solution was recorded on the Bruker AVANCE 400 spectrometer at 105.24 MHz and 162.0 MHz, respectively, with 85% H3PO4 and VOCl3 as external standards.
The 51V and 31P NMR spectra of the HPA-10 solution contains groups of lines typical of Keggin-type anions Hx−1PVxMo12−x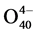 (HPAn-x, x = 1 - 5) [13] . In addition, the 31P NMR spectrum shows a separate peak of H3PO4. Besides, there is a broad signal of cation
(HPAn-x, x = 1 - 5) [13] . In addition, the 31P NMR spectrum shows a separate peak of H3PO4. Besides, there is a broad signal of cation  at 530 - 560 ppm overlapping HPAn-x lines in the 51V NMR spectrum (in more detail see [13] [14] .
at 530 - 560 ppm overlapping HPAn-x lines in the 51V NMR spectrum (in more detail see [13] [14] .
The atomic P:(Mo + V) ratio is higher than 1/12 in this non-Keggin HPA-10 solution. Therefore, it contains extra H3PO4, and the HPA-10 solution is actually a mixture of H3PO4 with different Keggin-type acids. But only for short we write the composition of the HPA-10 solutions as H17P3Mo16V10O89.
Thermal stability of the HPA-10 solution was studied by running some cycles of reduction of V(V) to V(IV) by 10 M N2H4·H2O at 100˚C [15] followed by its regeneration with O2 at different temperatures and РО2 = 4 at (405 kPa). The results of many-cycled tests shown that 0.2 M HPA-10 solution are stable up to 170˚C. It means that it can be successfully regenerated at 150˚C - 170˚C with retaining its homogeneity. This solution is quite promising as a bifunctional catalyst.
HPLC method was applied for reaction product analysis. For the purpose we used liquid chromatograph Pro-Star equipped with UV-detector (wavelength 247 nm). Separation was done on column Pursuit 3 C18, 247 × 4.6 mm, eluting reagent flow rate being 1 ml/min. We used commercial available solvents for the chromatography without further purification. Eluting composition was 70% CH3OH + 30% CF3COOH, sample solvent CHCl3.
All substituted AQ were identified by chromatography-mass spectrometry and comparison their HPLC retention times with commercial samples.
Substituted AQ were synthesized in a thermostat glass reactor by introducing substrate (NQ or 6,7-dimethyl-NQ) in amount of 0.2 g. Then 8 ml of organic solvent were added under stirring. After substrate dissolving, 0.2 M aqueous solution of HPA-10 (H17P3Mo16V10O89) and diene were added in required amount. After that reactor jacket was connected to thermostat preliminary heated to 80˚C. Reaction mixture was vigorously mixed with magnetic stirrer (650 min−1) for 7 hours. In the synthesis course HPA changed its color from dark red to green, while reaction product precipitated.
After process completion reaction mixture was twice diluted by water, solid precipitate was filtered away, washed with water to neutral pH and dried in vacuum over P2O5. After drying precipitate was weighted and analyzed with HPLC.
The substituted AQ yield was calculated according to the formula:

where M is the dry product mass, g; CAQ is the substituted AQ portion in precipitate according to analysis; g—the theoretically possible value of substituted AQ mass (for full NQ conversion into AQ), g.
After solvent and water access were distilled away (dioxane azeotrope with water boiled at 88˚C), reduced HPA-10 was regenerated by oxygen according to procedure described elsewhere [16] and many timed reused. No catalyst regeneration was performed, when diglyme was used as solvent (tb = 162˚C).
3. Results and Discussion
In the present work, we have synthesized substituted anthraquinones in the presence of aqueous HPA-10 solution according to scheme:

Synthesis conditions and experimental results are given in Table 1.
NQ reaction with methylsubstituted butadienes—isoprene (2-methylbutadiene) and trans-piperylene (trans-1- methylbutadiene)—yields corresponding methylanthraquinones under conditions described elsewhere [11] . In case of trans-piperylene reaction goes easily with a rather high yield (84%) giving 99% pure 1-methylanthraquinones. As for isoprene dissolved in dioxane, reaction produces a rather pure corresponding anthraquinone (98%), but its yield is not high (50%).
NQ and 6,7-dimethyl-NQ interaction with 2,3-dimethylbutadiene proceeds smoothly, giving 2,3-dimethylanthraquinone and 2,3,6,7-tetramethylanthraquinone with yields 78% and 70%, respectively. The worst results are related to chloroprene (2-chlorobutadiene), since chloroanthraquinone yield does not exceed 30%.
In order to increase the yield of desired products we have varied the conditions for the synthesis of 2-methyland 2-chloroanthraquinones (see Table 2). Apparently, within the tried reaction conditions we have failed to increase the yield of 2-chloroanthraquinone.
However, in case of 2-methylanthraquinone dioxane substitution by diglyme allows to increase the product yield up to 80% (Table 2). Note also that as molar ratio HPA-10: NQ decreases from 2:1 tо 0.5:1 (theoretically ratio 0.4:1 is enough for the complete diene synthesis adduct oxidation to AQ) 2-methylanthraquinone yield increases but not decreases. Similar result we observe for 1-methylanthraquinone. Most likely this is caused by the less product loss due to the side oxidation processes occurring in the excess of HPA, which is a rather strong oxidant with solution redox potential of about 1.0 V [17] . Therefore one may essentially reduce catalyst weight in case of substituted AQ, thus using molar ratio substrate: HPA lower than 2:1. This should allow the higher efficiency of developed one-pot processes for the synthesis of substituted AQ.
4. Conclusions
The one-pot synthesis of substituted 9,10-anthraquinones by reactions of 1,4-naphthaquinone and 6,7-dimethyl- 1,4-naphthaquinone with substituted 1,3-butadienes in the presence of aqueous HPA-10 solutions mixed with organic solvents has been developed. This method is proved to produce substituted anthraquinones with the 90% yield and 96% - 97% purity.
The obtained results open perspective for the low waste single stage processes for substituted AQ production

Table 1. Experimental results related to the diene synthesis of substituted anthraquinones.
Reaction conditions: 15.6 ml of 0.2 М aqueous solution of HPA-10, molar ratio HPA-10/NQ = 2, organic solvent (1,4-dioxane) volume 8 ml, reaction time 7 h, temperature 80˚C.

Table 2. Reaction parameters influence on the yield and purity of substituted methylanthraquinones.
Reaction conditions: 0.2 g NQ, 0.2 М aqueous solution of HPA-10, organic solvent volume 8 ml, reaction time 7 h, temperature 80˚C.
from substituted 1,3-butadienes and NQ in the presence of Mo-V-P HPA solutions being bi-functional (acid and oxidation) catalysts.
NOTES
*Corresponding author.