Preparation of Microcapsules Containing Aqueous Solution of Azur B with Melting Dispersion Cooling Method and Application to DNA Amplification Detector ()

1. Introduction
Microcapsules have various functions such as protection and isolation of core material, instantaneous and controlled release of core material, surface modification of core material, solidification of liquid and gaseous core materials, responsibility to various stimuli and so on [1] [2] .
Thus, the microcapsules have prepared mainly with both the chemical and the physicochemical methods and have been applied in the various fields such as cosmetics [3] , paintings [4] [5] , adhesive [6] , medical and dental supplies [7] [8] , information recording materials [9] , food materials [10] -[12] , agricultural materials [13] , drugs [14] [15] and so on.
Stimuli-responsibility of their functions is the most important one, because the core materials are able to be released according to the stimulus species such as mechanical pressure [16] , temperature [17] , pH of solution [18] [19] , specific enzyme [20] .
Azur B of a water soluble dye is known to singularly react with plant DNA and to be applied to detect plant DNA, namely, to utilize as the Amplification Detector of plant DNA [21] [22] .
If the aqueous solution of Azur B could be microencapsulated with the shell material to reveal the thermal responsibility and these microcapsules could be dispersed stably in the aqueous solution of plant DNA, the concentration of DNA in the aqueous solution should be able to be detected instantaneously by destroying the microcapsules due to heating and releasing the aqueous solution of Azur B without direct contact to the detecting system. For this purpose, it is necessary to prepare the microcapsules by the shell material with the sharp responsibility to the desired temperature and the perfect water proof.
In this study, paraffin wax is selected as the shell material to satisfy these demands and the melting dispersion cooling method is adopted to prepare the microcapsules [20] . In this microencapsulation process, it is extremely important to form the stable multiple emulsion.
In general, the (W/O)/W emulsion as the multiple emulsion has been utilized for preparing the microcapsules containing the aqueous solution [13] [16] . However, when the core material is water soluble or hydrophilic, the water phase is not suitable to the continuous phase, because the core material is easily leaked from the shell particle in the microencapsulation process. As a result, the content of core material should be decreased. Taking these things into consideration, the silicon oil as the stronger hydrophobic material is adopted as the continuous phase in this study.
The purposes of this study are to develop the method for preparing the microcapsules containing the aqueous solution of water soluble dye, to investigate the effects of the preparation conditions on the microencapsulation efficiency, the content of aqueous solution and the thermal responsibility and to apply to the DNA amplification detector.
2. Experimental
2.1. Materials
The materials used in this study are as follows. Paraffin wax (O) with melting temperature of 75˚C was used as the shell material. Azur B of a water soluble dye with molecular structure as shown in Figure 1 was used as the core material, which is known to singularly react with plant DNA.
The silicon oil (KF400, Shinetsu Chemical Co, Ltd) was used as the continuous phase (O’). As the oil soluble surfactants, Poem PR-100 and Poem J0021 (Riken Vitamin Co, Ltd) were added in the melted shell material to form the (W/O) dispersion and in the continuous phase to form the (W/O)/O’ emulsion, respectively.
2.2. Preparation of Microcapsules
Figure 2 shows the flow chart for preparing the microcapsules. The aqueous solution (W) dissolving the given amount of Azur B was dispersed in paraffin wax (O) by stirring with the rotor-stator homogenizer to form the (W/O) emulsion. Then, this (W/O) emulsion was dispersed in the continuous phase of silicon oil (O’) by stirring with the six bladed disc turbine impeller to form the (W/O)/O’ emulsion. This operation was conducted at temperature of 85˚C to melt paraffin wax.
When the (W/O)/O’ emulsion was cooled down to 50˚C, the microcapsules were prepared due to solidification of paraffin wax of the shell material. In this preparation process, the volume of aqueous solution of Azur B in paraffin wax (hold up in the (W/O) emulsion) and the concentration (Cs) of oil soluble surfactant (PoemPR- 100) in the (W/O) emulsion were changed stepwise. The experimental conditions are shown in Table 1. Here, the hold up in the (W/O) emulsion is defined as the ratio of the volume of aqueous solution to the total volume
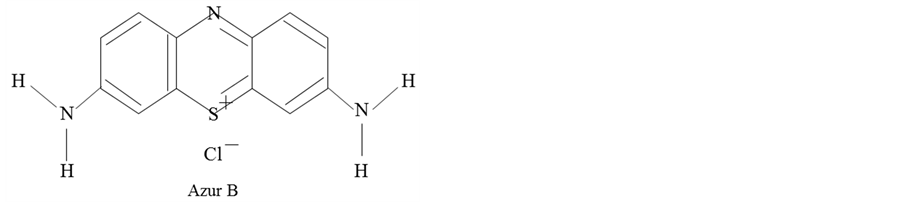
Figure 1. Molecular structure of Azur B..