1. Introduction
Fluorescence emission in the vicinity of thin metal films can excite and couple to the surface plasmon modes of the metals leading to highly directional wavelength resolved emission known as surface plasmon coupled emission (SPCE) [1]. This phenomenon has been used to develop sensing platforms promising far superior sensitivity and signal collection efficiency (>50% signal detection efficiency), in comparison to conventionally used detection methods such as fluorescence which is an isotropic phenomenon (<1% signal detection efficiency) [2-4]. Such platforms have already been used for the successful detection and sensing of many analytes both of biological and chemical in origin [5-9]. SPCE is a polarization dependent phenomenon where, only p-polarized incident light leads to the generation of the surface plasmons on the metal surface. The output signal is also highly p-polarized. Since the generation of surface plasmon polaritons only occurs at certain incident angles, the output emission is also observed to be highly directional at a particular angle leading to greater efficiency in signal collection [1,3].
Metals like gold and silver are used for the fabrication of thin films with silver being the preferred choice due to its property of greater absorption of incident light or energy [10,11]. However silver is highly prone to oxidation leading to fluorescence quenching at its surface [1,12]. To prevent the oxidative damage of its surface, silver is usually coated with a protective layer known as a spacer layer. Conventionally SiO2 has been used as a spacer layer material which has no role in the generation of the SPCE signal [1,2]. Different oxide materials are now being tested to perform the role of a spacer layer with other advantageous properties. Nano oxides are highly stable and have a mesoporous structure with pores in the nano-regime. The mesoporous nature of such materials results in the exhibition of an enormous surface area and the innate roughness presented at their surface, enabling greater binding to molecules [13]. Motivated by this fact, we have chosen to engineer and fabricate silver based SPCE substrates with a nano α-alumina coat which would lead to greater binding of the dye molecules leading to a greater signal yield. The coat of alumina on silver also renders the surface, highly inert to the thermal and chemical variations which may lead to attenuation of the SPCE signal.
On fabricating such thin films, we have performed SPCE studies involving measurement of enhancement, polarization and directionality of the coupled emission. The fabricated substrates were subsequently extended to the sensing of the toxic Cd2+ ions in an aqueous solution. This is a current relevant problem, as toxic heavy metal ion contamination is observed in soil and ground water resources [14].
2. Experimental
Thermal evaporation was used to fabricate 50 ± 2 nm Ag thin films on a SiO2 substrate [1]. The thickness was monitored using a quartz profilometer.
20 g of aluminium nitrate-Al(NO3)3·9H2O and 8 g urea, were dissolved in 15 ml distilled water and 0.2% Tween 20. The mixture was introduced into a muffle furnace at 500˚C for combustion synthesis [13]. On boiling and foaming, the mixture ignites with an incandescent flame leading to the formation of the final product nano α- alumina in 3 minutes. The product was characterized using a powder x-ray diffractometer and FESEM.
A 1% aqueous PVA polymer solution was prepared. A 1 mM stock solution of Rhodamine B (RhB) was prepared by adding 4.70 mg of RhB to 10 ml water which was serially diluted to prepare a 10 μM fluorophore solution. The synthesized α-alumina was dispersed into the RhB-PVA matrix on tip sonication. The resultant solution was spin coated on to the fabricated 50 nm Ag slides to form a nano alumina oxide doped RhB-PVA overcoat of 30 ± 5 nm [1,2] to form the SPCE substrate.
A 1 μM aqueous solution of Cd2+ ions was prepared by diluting 1 M solution of cadmium chloride and successively diluting it to the required concentration. The prepared 1 μM solution of Cd2+ ions was spin coated onto the -alumina-RhB-PVA coated silver substrate. Another silver substrate was coated with only water to serve as the control slide.
The slides were affixed to a BK7 hemi cylindrical prism placed on a 360˚ rotary stage for performing the SPCE studies in the Reverse Kretschmann (RK) configuration [1]. A 532 nm CW laser was used for illuminating the sample and the emission @ 580 nm was recorded. The Ocean optics© USB4000 spectrometer was used for detection of the SPCE signal which is based on a fiber optic probe. The experimental schematic is depicted in Figure 1.
The measurements involve recording and determination of the enhancement, % p-polarization and directionality of the coupled emission, using a fiber optic probe.
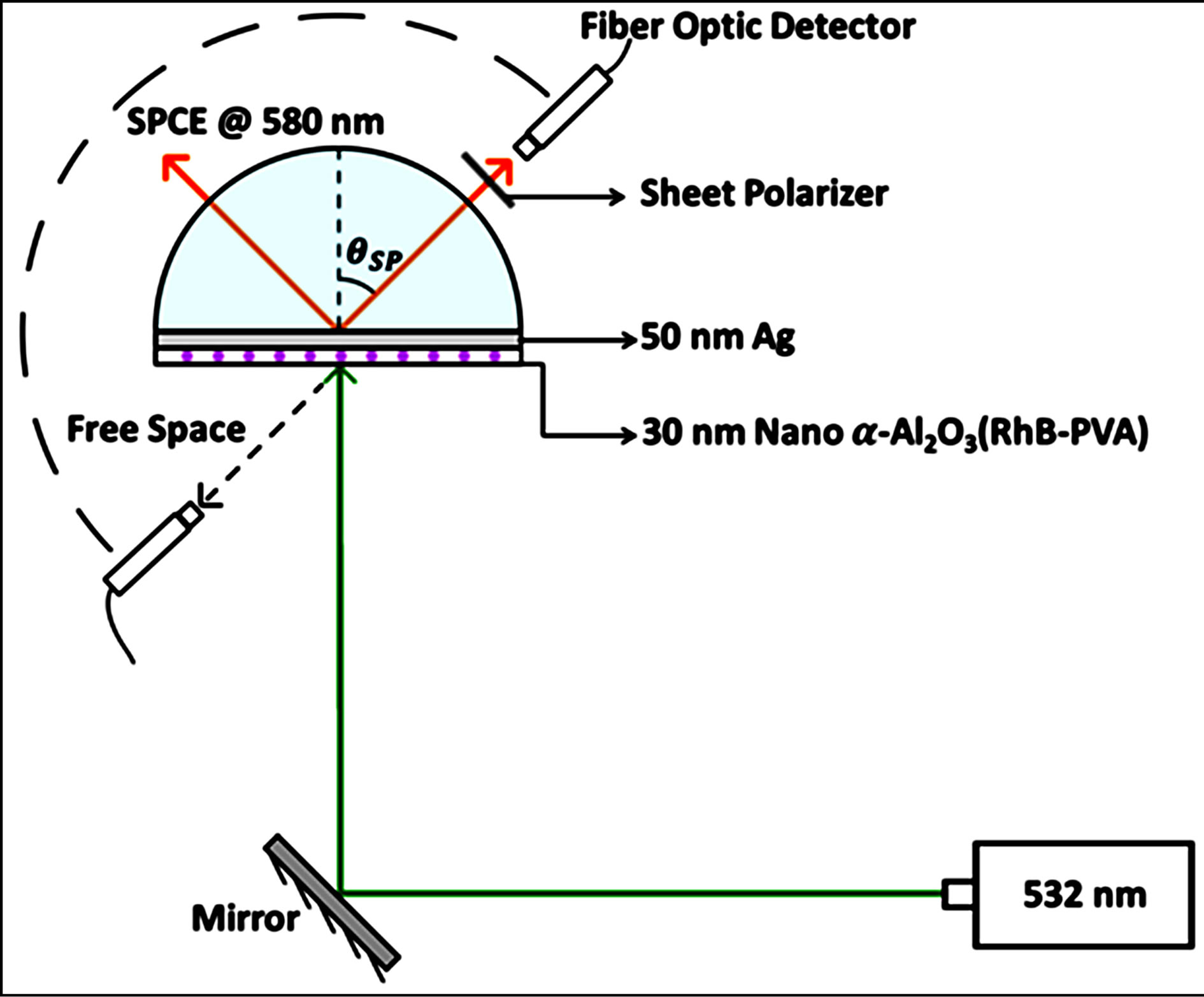
Figure 1. Schematic of the SPCE experimental platform consisting of 532 nm excitation source and collection of coupled emission @ 580 nm, generated at the θSP (surface plasmon angle).
The ratio of the recorded intensity of the SPCE signal to the isotropic free space emission corresponds to the degree of enhancement achieved, and the angle at which maximum emission is observed, corresponds to the directionality measurements. In a similar manner, the fiber optic probe is used to record the intensity of the “P” versus “S” polarized emission, corresponding to the polarization measurements.
The SPCE measurements were first performed on the nano α-alumina-silver, thin film stack, before being extended to the detection of the Cd2+ ions. The intensity of the SPCE signal arising from the experimental thin film stack was monitored on exposure to the aqueous solution of cadmium ions.
Theoretical simulations based on the SPR theory were performed on the TFCalc© software platform (Software Spectra Inc., Portland, OR) [1,2], to determine the angle of minimum reflectivity which correlates to the angle at which the SPCE signal would be observed. The angle of minimum reflectivity or the SPR angle is highly specific to the thickness of the substrate. The minimum reflectivity curve was generated for 50 nm Ag with a 30 nm RhB-PVA overcoat. The experimentally observed angularity of the directional emission can be correlated to the theoretically generated angle of minimum reflectivity which acts as a tool to confirm the thickness of the fabricated substrate [1,2].
3. Results & Discussion
The synthesized aluminium oxide was subjected to XRD analysis, Figure 2(a) displays the XRD pattern corresponding to the α-Al2O3 phase. FESEM was used to determine the particle size of the nano α-alumina; Figure
 (a)
(a) (b)
(b)
Figure 2. (a) XRD pattern for nano α-alumina; (b) FESEM image of nano α-alumina.
2(b).
An “8” fold signal enhancement was observed on calculation of the ratio between the SPCE signal and the isotropic free space emission; Figure 3(a). Corresponding studies performed on RhB coated Ag substrates, without the oxide coating, only generated a “5” fold signal enhancement, which substantiates the theory proposed, that the high surface area of the nano α-alumina leads to a greater adsorption of the RhB resulting in a higher emission intensity.
Polarization measurements were performed by recording the SPCE signal through a sheet polarizer placed in the horizontal (“H”/p-polarized) and vertical (“V”/spolarized) configurations. The percentage p-polarization of the emitted signal was determined as follows [2]:



Figure 3. (a) Enhancement plot for nano α-alumina doped RhB showing “8” fold enhancement; (b) Polarized emission intensity plot showing predominantly (92%) p-polarized output.
where, I is the intensity of the horizontal or vertically polarized light. A 92% p-polarized output was observed indicating a strong coupling of horizontally polarized light in accordance with the theory of SPCE Figure 3(b).
The angle corresponding to the observation of maximum signal intensity on the SPCE side of the prism is recorded as the angularity data, which can be correlated to the angle of directional emission in the theory of SPCE. The maximum SPCE signal intensity was observed @ 49˚ corresponding to the angularity of directional coupled emission; Figure 4(a). The observed angularity (49˚) was in coherence with theoretical calculations performed using the TFCalc software to determine the angle of minimum reflectivity or the surface plasmon angle, (49.2˚); Figure 4(b).This strong correlation confirms the 50 nm Ag + 30 nm nano α-alumina-RhB-PVA thickness of the fabricated substrate.
The detection of the Cd2+ ions was performed by monitoring the behavior of the enhancement profile of the SPCE signal on exposure to the cadmium ions. Comparison between the recorded intensity of the coupled
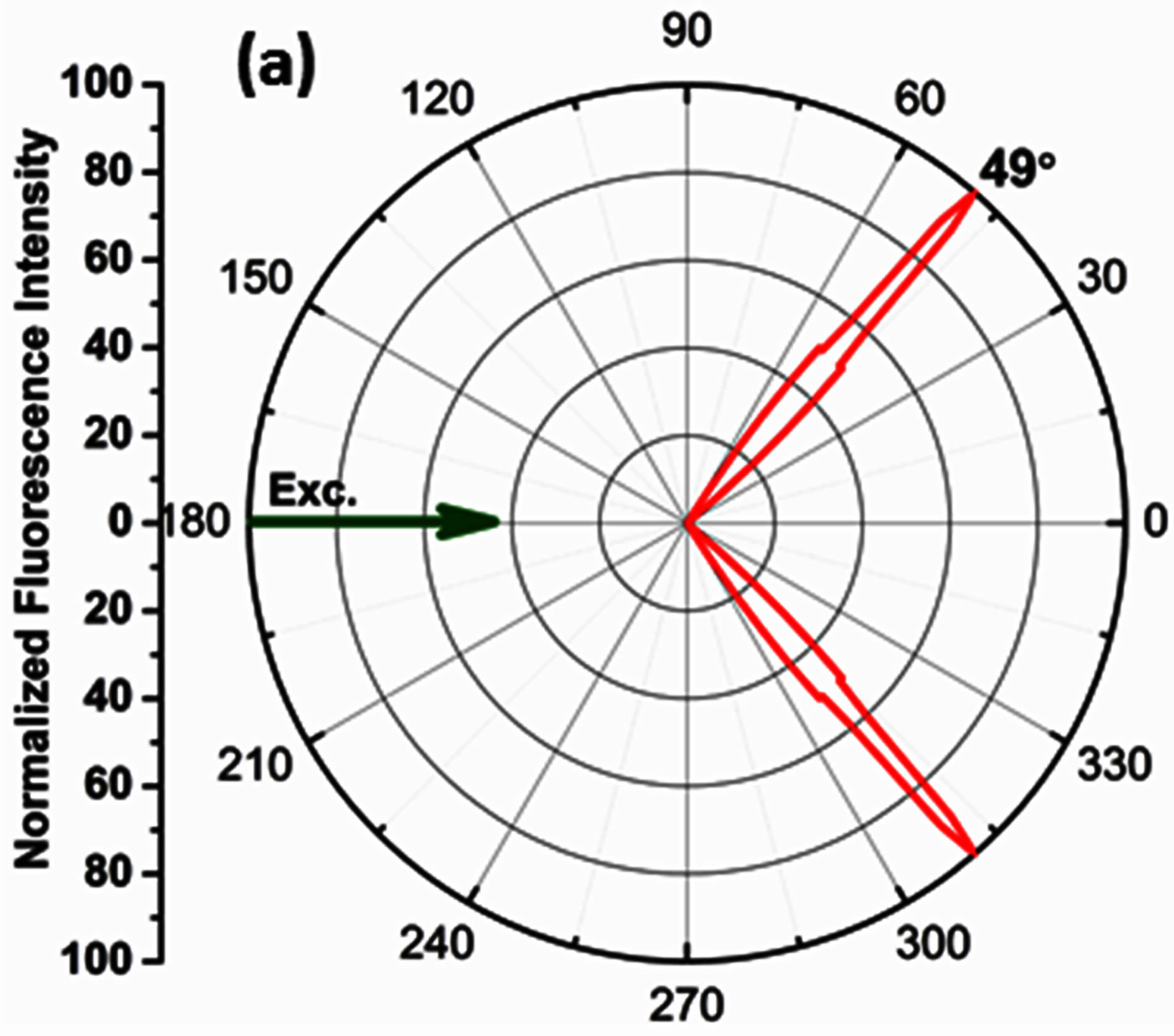

Figure 4. (a) Radial plot displaying directional emission observed @ 49˚ (b) TFCalc software generated reflectivity curve, showing angle of minimum reflectivity @ 49.2˚.
emission signal, arising from the slide exposed to the cadmium ions versus, intensity from the control slide which was coated with only water, was performed. The purpose of the control slide is to rule out any role, which water may play in the alteration of the intensity of the coupled emission signal. On recording the SPCE signal from the two slides it was observed that, the intensity of signal arising from the control slide did not show any significant change. However, the intensity of coupled emission signal arising from the slide exposed to the Cd2+ ions decreased by 50%. The coating of the Cd2+ ions led to significant attenuation or quenching of the SPCE signal by half, which can be considered as an indicator for the detection of the Cd2+ ions in this experiment; depicted in Figure 5.
We propose the interaction of the positively charged Cd2+ ions with the excited RhB species as the lead cause
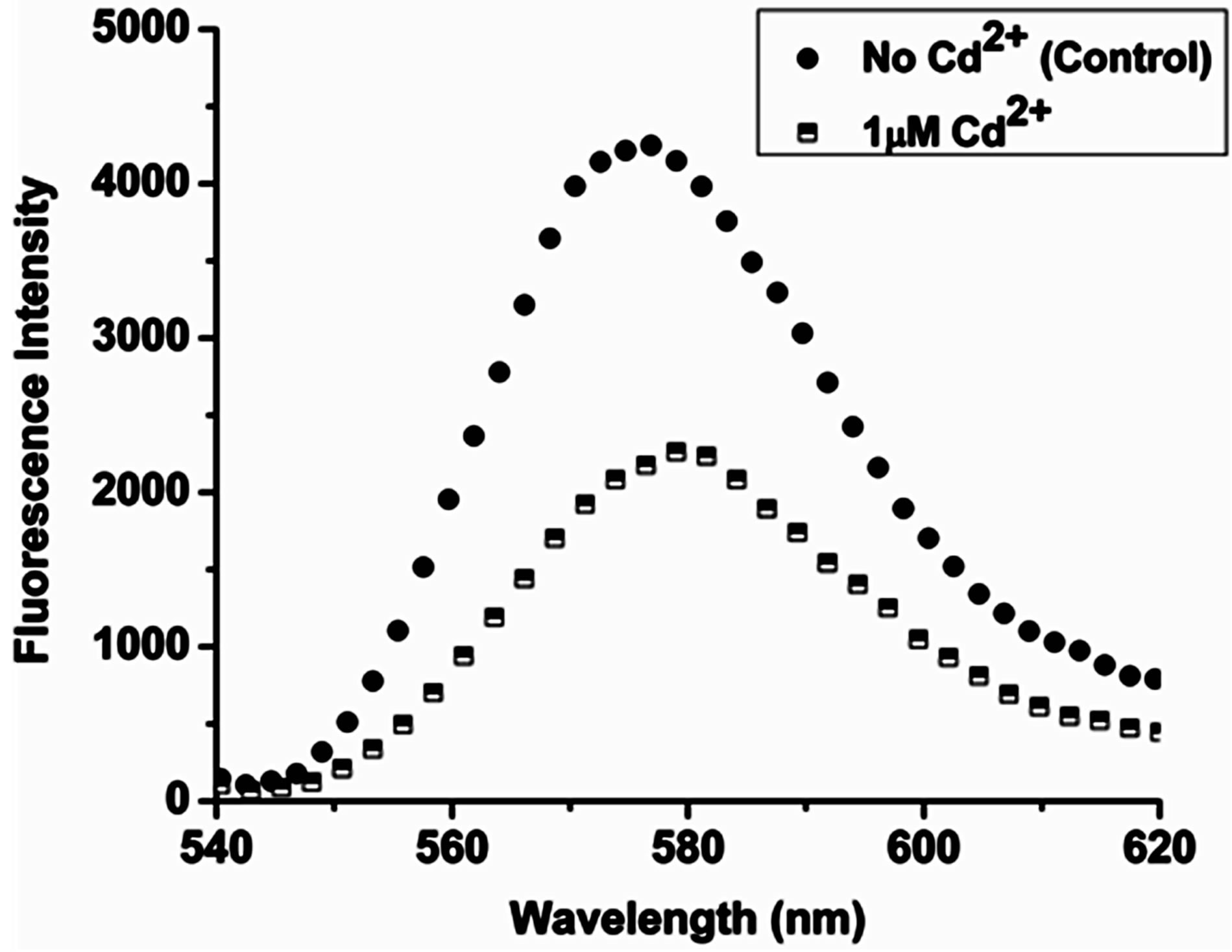
Figure 5. Fluorescence intensity plot displaying 50% signal attenuation for the experimental substrate, on exposure to 1 μΜ Cd2+ ions versus intensity of the control substrate.
in the drastic attenuation of the SPCE signal. The energy of the excited fluorophore species would be absorbed by the cadmium ions leading to inefficient relaxation of the excited fluorophores. This results in an overall decrease in the intensity of the observed coupled emission. Cadmium being a heavy metal dispersed on the RhB-PVA matrix, blocks the excitation of the fluorophores by shielding them from the incident excitation source.
4. Conclusion
The incorporation of nano -alumina into the RhB-PVA matrix led to an “8” fold enhancement in the SPCE signal intensity which can be attributed to the high surface area enabled superior adsorption of RhB at the surface of the substrate. The presence of the nano oxide layer also ensures the arrest of oxidation at the surface of silver thereby preventing any quenching of fluorescence. The fabricated substrates were successfully implemented towards the detection of Cd2+ ions in an aqueous medium, also ruling out the role of water in the detection process with the help of a control substrate. Cadmium ions quench the fluorescence from the RhB molecules by absorbing the energy of excitation from the fluorophores as well as, the energy from the source of incident excitation. Such platforms based on the SPCE phenomenon can be extended to the simple detection of different chemical or biological analytes. The detection can be made more specific by the prior functionalization of the substrates with molecules having a high affinity towards the analytes which forms our future work.
5. Acknowledgements
We thank Bhagawan Sri SathyaSai Baba, Founder Chancellor of the Sri SathyaSai Institute of Higher Learning, for being the source of constant inspiration and encouragement. We would also like to thank the DST (Inspire Fellowship Program), Govt. of India, for offering support. Our thanks to Prof. Lakshminarayanan V. and Mr. Dhason A., Raman Research Institute, for their help in characterization.