The Mechanism of Formation of Glass-Ionomer Cement: A Theoretical Study ()
1. Introduction
For decades, dentistry has sought an aesthetic material to replace traditional restorative metallic amalgams, showing a wear resistance comparable to the latter. In the quest for replacing amalgams, there have been two types of restaurateurs who use polymers, composites or glassionomers, each one of which, differently, meets most of the requirements of successful restorative materials [1]. The term glass-ionomer cement is reserved exclusively for a material consisting of a glass that breaks into an acid, and an acid which is soluble in water is a polyelectrolyte, which is formed by a neutralization acid-base reaction. The simplest form of these materials is considered an ionomer, a conventional glass. The term includes both polyalkenoate as polyphosphonate glasses [2].
The hybrid dental cements partially formed via an acid-base reaction or via a redox or photochemical (visible light) polymerization process are known as resinmodified glass-ionomers (RMGI). Conventional glassionomer cements (polyalkenoates) are formed by the reaction of poly (alkenoic acids), i.e. polyelectrolytes acid derivatives with a calcium-fluoroaluminosilicate (CaFAlSi) glass powder, using as the most acid monomers, acrylic acid (AA), methacrylic acid (MAA), itaconic acid (IA), maleic acid (MA), and 2-butene-1,2,3-tricarboxylic acid (BTA).
Resin-modified Glass-ionomer cements (RMGIC) are hybrid materials which not only experience an acid-base reaction, but also participate in a free radical polymerization because they contain polymerizable methacrylate groups in the polyacid skeleton embedded as side chains. These cements have more attractive properties compared with conventional GIC. The RMGIC diminishes the problems with moisture sensitivity and with low mechanical strength associated to the GIC. They are easier to manipulate clinically, showing a longer working time and having significantly improved some of the mechanical forces, such as flexural strength and diametral tensile [3].
The polymeric materials currently used for GIC are based on poly (acrylic acid), poly (acrylic acid co-itaconic acid) or poly (acrylic acid co-maleic acid). For visible light-cured (VLC) GIC, the polymer material has been chemically modified so it has double bonds polymerizable by free radicals; the aqueous formulation also contains a monomer with methacrylate groups. New routes have been explored for new copolymers of acrylic acid in order to improve both conventional and VLC GIC, where the acid groups are available for the formation of salt bridges. In particular, copolymers of acrylic acid modified with amino acids have been prepared [4]. 2-Hydroxyethyl methacrylate (HEMA) has been used as a major component in RMGIC because it acts both as co-solvent and co-monomer to increase the solubility in water of vinyl-containing polyacids. But it is known that HEMA shows potential cytotoxicity to pulp and surrounding tissue. Some studies have shown that RMGI systems without HEMA, based on amino acid derivatives, are better as dental restoratives. RMGI system is showing improvements in the mechanical strength and in the elimination of cytotoxicity caused by leaching of HEMA, the suggested derivative was acrylol target beta-alanine (MBA) [5].
The use of amino acid derivatives in the synthesis of resin-modified dental cements provides several advantages such as: 1) to allow greater adhesion of the material to tooth structure due to the free amide and other more residues of acidic groups, and 2) to maintain excellent biocompatibility because many of the amino acids that can be used are essential, i.e., they are part of the human body [6,7].
Despite the importance of the formation reactions of terpolymers for the construction of the cement and the technological significance of their production, the underlying mechanism is poorly understood. Therefore, we have performed density functional calculations to obtain detailed insight into these reactions and to identify the most likely reaction routes. Curiously, to the best of our knowledge, this is the first study aimed at investigating the reaction mechanism theoretically. We have selected the resin-modified glass-ionomer cement (RMGIC) as a typical and technologically relevant product, which is formed by acrylic acid (AA), itaconic acid (IA) and an amino acid derivative (AAD) obtained with acryloyl chloride and one of two amino acids: phenylalanine or tyrosine. The pendant methacrylate group (MPD) is grafted through glycidyl methacrylate (GM), and an intramolecular complex of aluminum tricarboxylate is studied. We have calculated the full Gibbs energy profiles of the possible reaction channels. Our main motivetions have been to identify the feasible reaction paths, to select intermediates that can be promising candidates for experimental detection and to provide a detailed mechanistic picture of the process that may help to develop more effective and environmentally benign versions of these types of chemical reactions for preparing electronic versions of their papers.
2. Computational Details
All calculations were carried out using the Gaussian03 computational package [8]. The geometric parameters for all the reactants, transition states and products of the reactions studied were fully optimized using density functional theory (DFT) [9] with B3LYP [10] functional and the 6-311G(d,p) basis set [11].
B3LYP is a combination of Becke’s three parameter hybrid exchange functional [12] with the Lee, Yang and Parr correlated functional [13]. All stable molecules and transition state (TS) structures have been verified by vibrational analysis. Additional IRC and normal optimization calculations always showed that the calculated TSs connect the two minima characterizing the elementary step in question. Thermal corrections to enthalpy and entropy values have been evaluated at the experimental temperatures T = 333.15 K to form the copolymer and T = 313.15 K for the addition of glycidyl methacrylate (GM). To calculate enthalpy and entropy values at a temperature T, the difference between the values at that temperature and 0 K has been evaluated according to standard thermodynamics [14].
3. Results and Discussion
The starting point of our investigation was the analysis of resin-modified glass-ionomer cement (RMGIC), which revealed two essential transformations constituting the pathway: the first one is the reaction of fixation of poly (alkenoic acid) among acrylic acid (AA), itaconic acid (IA), and the amino acid derivative (AAD), followed by the reaction with glycidyl methacrylate (GM) to graft the methacrylate group and so this molecule may subsequently be involved in a free-radical polymerization; third, we propose a possible reaction mechanism to get two different grafted copolymers and, finally, we show a possible intramolecular complex between the most stable grafted polymers and Al3+.
3.1. Reaction of Fixation of Poly (Alkenoic Acid) Abbreviations and Acronyms
We have studied three possible reaction sequences (see Figure 1) to obtain first AA-IA-AAD, which is formed by acrylic acid, itaconic acid, and the amino acid derivative in this order. The second one, by acrylic acid, the amino acid derivative, and itaconic acid (AA-AADIA), and the last one by the amino acid derivative, acrilyc acid, and itaconic acid (AAD-AA-IA). The different positions represent all possibilities of combination among these three monomers. It is important to clarify that due to that this process is a radical reaction, it is necessary to “close” the formed polymer with two methyl radicals which are marked in bold in Figure 1.
The optimized geometries of the three copolymers and their Gibbs energies related to the terpolymer AAD-AA-IA, which is the most stable one, are show in Figure 2. In this Figure only are draw the optimized geometries with the amino acid derivative with phenylalanine, the values of Gibbs energies for tyrosine polymers are in parentheses.
From a thermodynamic point of view is unlikely to

Figure 1. Three different possibilities of combination of monomers AA, IA, and AAD.

Figure 2. B3LYP/6-311G(d,p)-optimized geometries of the three copolymers with phenylalanine. Relative Gibbs energy values in kJ·mol−1, calculated at 333.15 K. Values in parentheses are those calculated for copolymers with tyrosine.
form the copolymer AA-AAD-IA, because its Gibbs energy is very high, 82.1 kJ·mol−1 (88.4 kJ·mol−1 for X = OH) related to IA-AA-AAD. An analysis of its optimized geometry shows a central carbon which try to get away the constituent monomers, this implies that not necessarily the location of the amino acid derivative between acrylic and itaconic acids makes that the carboxyl groups in the copolymer are more distant from each other. The other two possibilities (AA-IAAAD and AAD-AA-IA) have lower values for the Gibbs energy and therefore are more likely to be formed. For this reason, we decided to study the addition reaction of glycidyl methacrylate (GM) only to the other two polymers, AA-IA-AAD and AAD-AA-IA.
3.2. Reaction between Copolymer AA-IA-AAD or AAD-AA-IA and Glycidyl Methacrylate (GM) Units
GM can react with one of the carboxyl groups present in the copolymer, which are displayed in Figure 3, to form a grafted polymer (GP) with unsaturated bonds that can be used later in the process of light-curing. In this figure we only show the reaction between AA-IA-AAD and GM in position 1, to get the grafted polymer.
The grafted polymers obtained by GM, instead of IEM (2-Isocyanatoethyl methacrylate) as suggested by some authors, [7] have two pendant methacrylate groups (MDP) which can react after the light-cured process.
Due to the polymers shown in Figure 3 contain three different types of carboxylic acid groups, we can select the following names for the copolymers: 1 and 4 (acid C attached), 2 (acid CH attached), and 3 (acid CH2 attached), i.e., the number 1 is assigned if GM is added to the carboxylic group of AA, the numbers 2 and 3 if GM is added to the COOH or CH2COOH groups of IA, respectively, and the number 4 if GM is added to the carboxylic group of AAD.
The values of the Gibbs energies of the products formed by the addition of GM to AA-IA-AAD or AADAA-IA, in each one of carboxylic groups (the numbers correspond to those are shown in Figure 3) are collected in Table 1.
It is noted that the copolymer AA-IA-AAD produced grafted polymers with Gibbs energies more stable than AAD-AA-IA, for both compounds X = H, and X = OH. However, for X = H is more stable when GM joins in position 3, while for X = OH it is in position 4. This difference may be due to the presence of the OH group, yielding a larger steric hindrance.
The next study is devoted to analyze the reaction mechanism for the addition of GM to copolymer, and two different reaction mechanisms have been considered via six-membered cyclic transition states (TSs), like it is shown in Figure 4.
Figure 5 shows a schematic representation of the reaction pathways to clarify the movements of the different atoms involved in the TSs. In both proposed mechanisms, A and B, a water molecule is formed, and the corresponding TSs are cyclic, obtaining a polymer grafted with two or one methacrylate groups, respectively.
During the process, both transition states show that the H3 is already loose and ready to form the water molecule, but the distance is greater between H3-C4 in TS_B than in TS_A, indicating the lability of the hydrogen. Each transition state is characterized by one imaginary frequency of 999.1 cm−1 for TS_A and 566.4 cm−1 for TS_B. The mode corresponding to the imaginary frequencies can be described as lengthening C1-O2 and H3-C4 bonds and shortening of C1-O6 bond.

Table 1. Free energies of each one of grafted polymers calculated at B3LYP/6-311G(d,p) level of theory.

Figure 3. Scheme of reaction between carboxylic group numbered 1 in AA-IA-AAD and GM to get the grafted polymer (GP_1).
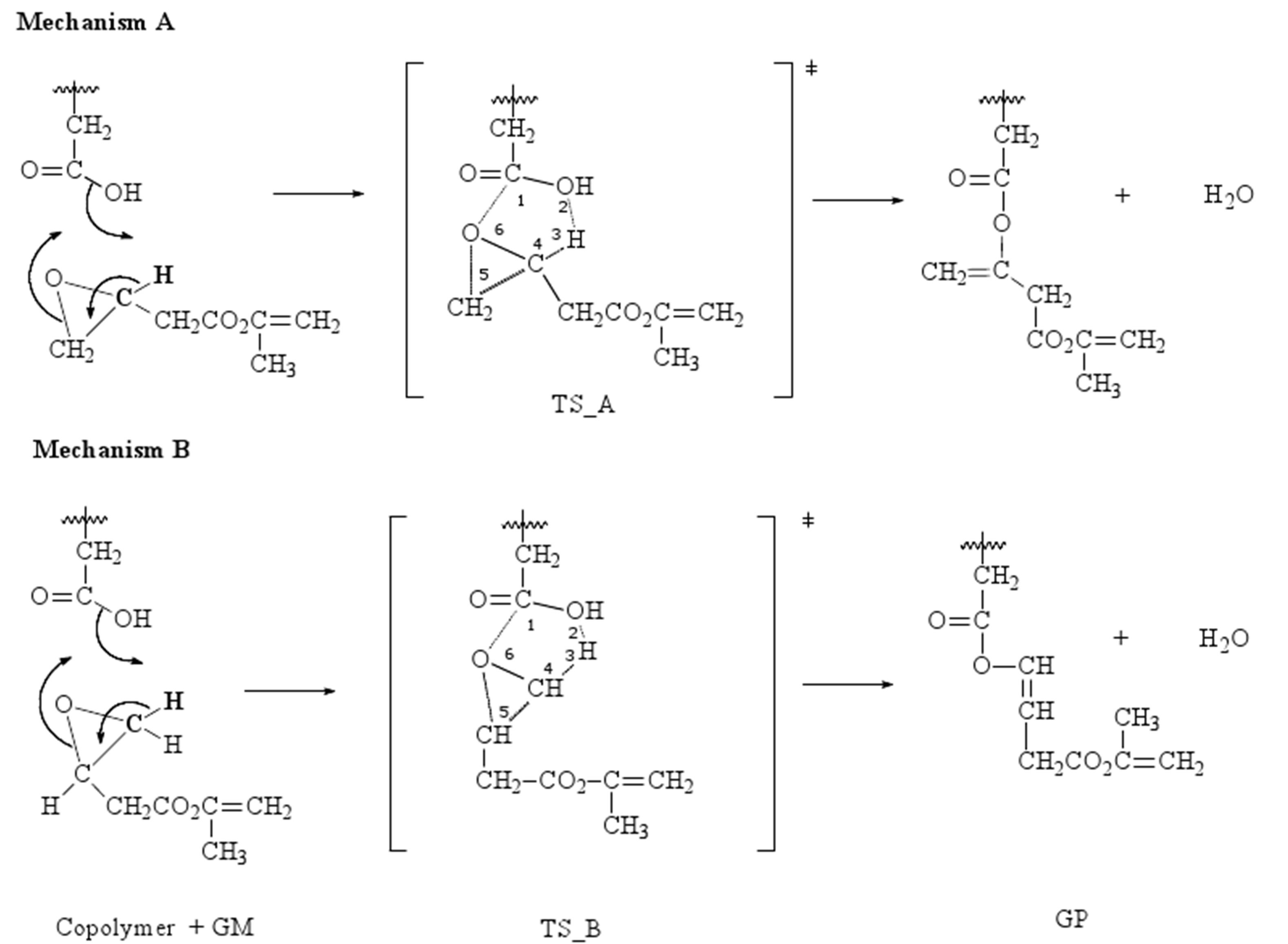
Figure 4. Proposed mechanisms for the addition of GM to copolymer.
The grafted polymers found with the second mechanism were optimized at the same level of theory only for copolymer AA-IA-AAD, and the Gibbs energies were obtained for each one of the four carboxylic groups: −15.3, −44.5, −51.5 and −15.7 kJ·mol−1 (for X = H) and 6.1, −29.8, −32.0 and 6.0 kJ·mol−1 (for X = OH), respecttively. These compounds are less stable than those found with the first mechanism, and we believe it is unlikely that these are formed.
3.3. Intramolecular Complex between Grafted Polymers and Al3+
A resin-modified glass-ionomer cement (RMGIC) also contains a glass powder calcium-fluoroaluminosilicate acting as the source of cross-linking through the formation of salt bridges in the acid-base reaction. But according to published studies, [15,16], not all the carboxylic groups of poly(acrylic acid) are converted to carboxylate groups during the course of the reaction. In fact, studies have shown that very few interor intramolecular Al3+ tricarboxylate complexes (salt-bridges) are formed, which is understandable in view of steric requirements.
In this study, we optimized two intramolecular Al3+ tricarboxylate complexes (salt-bridges) formed from the most stables grafted polymers, i.e., GP_3 for X = H and GP_4 for X = OH, and their optimized structures are shown in Figure 6.
As shown in Figure 6, in GP_4_Al, the structure is more compact due to there are also hydrogen-oxygen-
 TS_A
TS_A  TS_B
TS_B
Figure 5. B3LYP/6-311G(d,p)-optimized geometries of two transition states representing the mechanisms A and B.

Figure 6. B3LYP/6-311G(d,p)-optimized geometries of two intra-molecular Al3+ tricarboxylate complexes.
hydrogen distances shorter that can make the molecule more stable, and the methacrylate groups are more hindered for subsequent polymerization reaction, while GP_ 3_Al shows a looser structure and more potential for giving the subsequent reaction.
4. Conclusion
A theoretical study on the formation of a poly (alkenoic acid) and the possibility of its reaction with glycidyl methacrylate (GM) was carried out at the B3LYP/6- 311G(d,p) level of theory. We have considered the merger of three monomers: acrylic acid (AA), itaconic acid (IA) and an amino acid derivative (AAD). Based on our theoretical results, we conclude that although the copolymer AAD-AA-IA is the most stable, the copolymer AA-IA-AAD produced grafted polymers with Gibbs energy values more stable than the other two possible combinations. The grafted polymers obtained with GM have two pendant methacrylate groups (MDP) which can react after a light-cured process. Finally, we show how the structure GP_3_Al is looser, which would allow to act as a source of cross linking through the formation of salt-bridges in the acid-base reaction.
5. Acknowledgements
This work was partially financed by the “Fortalecimiento de Grupos” project of the Universidad Nacional de Colombia. E. Vélez especially gratefuls to Dr. Juan Andrés Bort for its valuable assistance in this work and for allowing that the calculations were made in the Universitat Jaume I, Castellón, Spain.