Atomic Absorption and Vibrational Spectral Magnetic Studies on the Removal of Cu(II) and Co(II) Ions Using Synthetic Nano Adsorbent Fe3O4 ()
1. Introduction
The daily requirement of copper is about 2.0 mg for an adult [1]. Even though copper is needed for all human beings, problems arise when it is deficient or in excess. The Environmental Protection Agency’s (EPA) lead and copper rule promulgated, in 1991, that the maximum contaminant level goal (MCLG) for copper [2] is 13 mg∙L−1. The presence of excess of copper causes Wilson’s disease [3] due to which the bio-synthesis of ceruloplasmin is suppressed. The ingestion of acute toxic levels of copper may result in nausea, vomiting, diarrhea, liver damage, hemoglobinuria and hematuria. Higher doses of cobalt can adversely affect the hemoglobin content of the patient’s blood and produce polycythemia and hyperlipemia [4]. Cobalt poisoning leads to gastrointestinal distress and heart failure. The hazardous nature of heavy metals (like copper and cobalt), even in trace quantities due to their tendency for chelation and accumulation with the organic and biomolecules, makes it necessary to develop suitable cost-effective methods for the removal of heavy metals from waste water systems and industrial effluents fed into water stream.
2. Experimental Procedure
Preparation of Nano Fe3O4
6.48 g of Ferrous Chloride and 4.78 g of Ferric Chloride dissolved in 200 mL of distilled water and 10% Polyethylene glycol together and dispersed by ultra sonic stirring for 10 min. The mixture was heated to 75˚C followed by the drop wise addition of 2 mol/L sodium hydroxide until the pH value of the mixture reached about 11.5. The mixing tested for 2 hrs at 60˚C and then aged at 80˚C for 30 min, purified and washed several times with distilled water at 80˚C [5,6].
3. Results and Discussion
3.1. X-Ray Analysis
Figure 1 (a and b) shows the X-Ray diffraction pattern of the commercial macro Fe3O4 and chemically synthesized Fe3O4 particles by co-precipitation of Fe2+ and Fe3+ ions in aqueous medium. The curve shows all the diffraction peaks of the Fe3O4 (JCPDS-75-1609) and the broadening of the diffracted peaks of the chemically synthesized Fe3O4 shows a growth of nanocrystalline phase with an average size of 20 nm calculated from the Debye-Scherer formula.
3.2. SEM Analysis
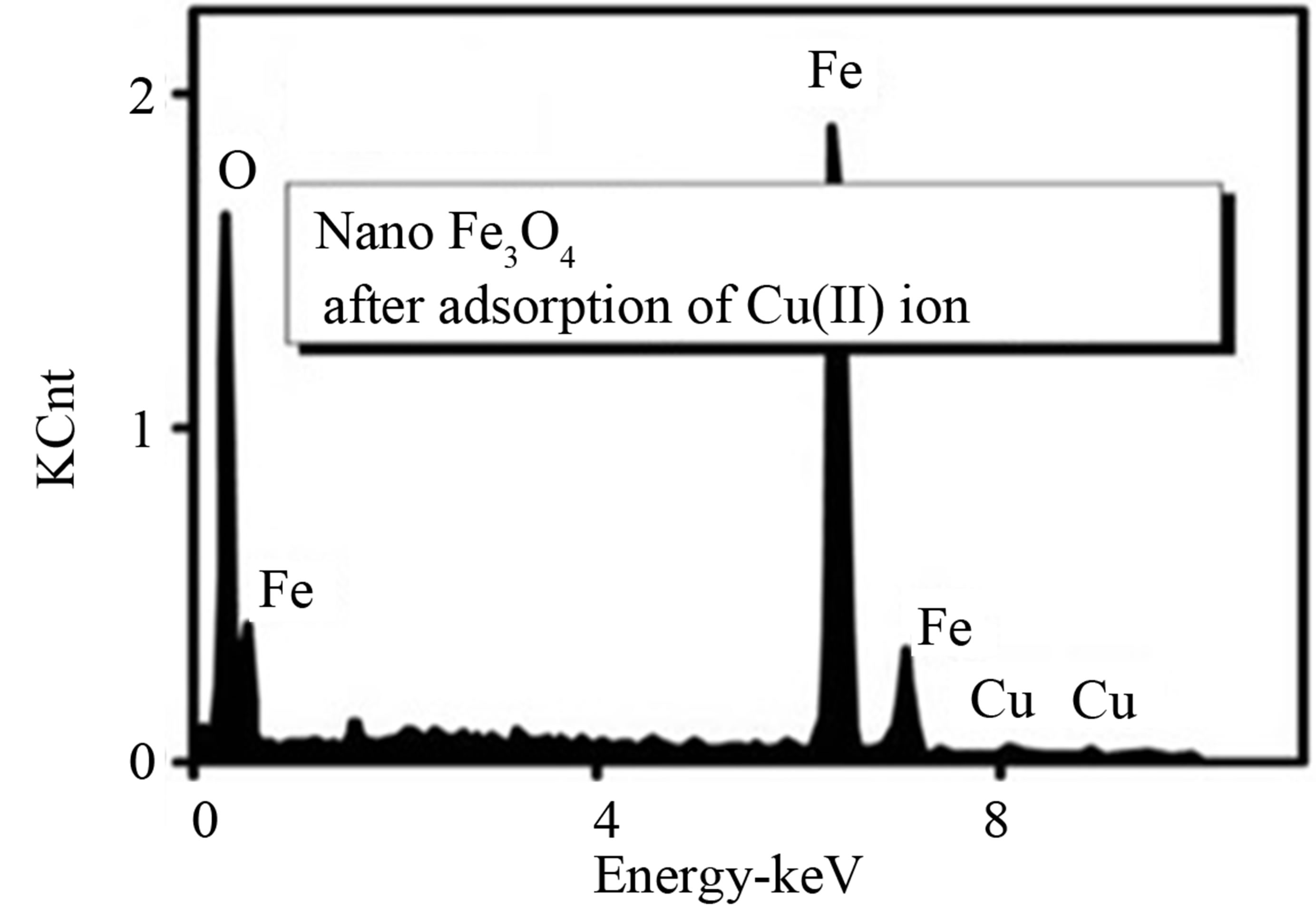
Figure 2 shows the high resolution scanning electron microscope (HRSEM) image of Fe3O4 and shows the formation of mild agllomeration with particles in the range of 18 - 23 nms. A X-ray energy dispersion analysis (EDAX) of the copper and cobalt solution treated Fe3O4 is depicted in Figures 3(a) and (b). From the EDAX spectrum, it is observed that there is major component of Fe and O due to Fe3O4 and presence of copper and cobalt adsorbed on it.
3.3. Effect of Initial Concentration
The adsorption experiment was carried out varying the concentration of Cu(II) and Co(II) ions (25, 50, 100, 150, 200, 250, 300, 350, 400 ppm) keeping the fixed dose of synthetic nano Fe3O4 (1.07g/L for Cu(II) ion, and 2.57 g/L for Co(II) ion), contact time (60 min), pH (5 - 6) and temperature 28˚C ± 1˚C. The variation in the percentage removal of Cu(II) and Co(II) ions with their concentration are shown diagrammatically in Figure 4. It was ob

Figure 1. XRD pattern of commmercial macro and synthesized nano Fe3O4.

Figure 2. SEM image of synthetic nano Fe3O4.
 (a)
(a) (b)
(b)
Figure 3. (a) and (b) EDAX for synthetic nano Fe3O4 after adsorption Cu(II) and Co(II).

Figure 4. Effect of initial concentration of Cu(II) and Co(II) ions on synthetic nano Fe3O4.
served that the percentage removal of Cu(II) and Co(II) ions by nano Fe3O4 is low at higher concentration and gradually increases as the concentration decreases. This is due to the fact that after the formation of mono-ionic layer at lower concentration over the adsorbent surface, further formation of layer is highly hindered at higher concentration due to interaction between Cu(II) and Co(II) surface and in the solution. In addition to that at low concentration of the Cu(II) and Co(II) ions, the ratio of the initial number of moles of the Cu(II) and Co(II) ions to the available surface area of the adsorbent is large and subsequently, the fraction of the adsorption becomes independent of the initial concentration of the metal ion. But at higher concentration, the adsorption sites available for adsorption become lesser, and hence, the percentage removal of the metal ions at higher concentration decreases [5,7]. The optimum concentration was found as 50 ppm for both Cu(II) and Co(II) ion with the effective removal of 97.8% for Cu(II) and 99.2% for Co(II) ions.
3.4. Effect of Dose Rate
The removal of the Cu(II) and Co(II) ions was studied with different dose of synthetic nano Fe3O4 from (0.82 to 2.07 g/L) at the optimum concentration of 50 ppm for both the metal ions with fixed contact time (60 min), pH (5 - 6) and temperature of 28˚C ± 1˚C. The effect of the dose rate of synthetic nano Fe3O4 on the removal of Cu(II) and Co(II) is pictured in Figure 5. It was noted that the percentage removal of the Cu(II) and Co(II) ion increases as the concentration of nano Fe3O4 increases owing to the enhanced total surface area of the adsorbent. This means that the toxic ions can be removed effectively from the contaminated water with the proper amount of the adsorbent, which would possess more adsorption sites available for the metal ion uptake from the solution [5,7]. The optimum dose rate was found to be 1.07 g/L for Cu(II) ion and 2.57 g/L for Co(II) ion with the effective removal of 97.8% for Cu(II) and 99.2% for Co(II) ions.
3.5. Effect of Contact Time
In order to study the effect of contact time on the removal of Cu(II) and Co(II) ions, experiment were conducted at different contact times (from 10 to 60 min) keeping the following conditions: optimum concentration (50 ppm for Cu(II) and Co(II) ions), fixed dose of synthetic nano Fe3O4 (1.07 g/L for Cu(II) ions, 2.57 g/L for Co(II) ions), pH (5 - 6) and temperature of 28˚C ± 1˚C. The variation of the percentage removal of Cu(II) and Co(II) ions by synthetic nano Fe3O4 with contact time was shown in Figure 6" target="_self"> Figure 6. The extent removal of Cu(II) and Co(II) ion increases sharply initially and found to be constant after the optimum contact time. The optimum contact time was found to be 60 minutes for Cu(II) ions and 10 minutes for Co(II) ions with the effective adsorption of 97.8% for Cu(II) and 99.2% for Co(II) ions.

Figure 5. Effect of the dose rate in the percentage removal of the Cu(II) and Co(II) ions by adsorption on synthetic nano Fe3O4.
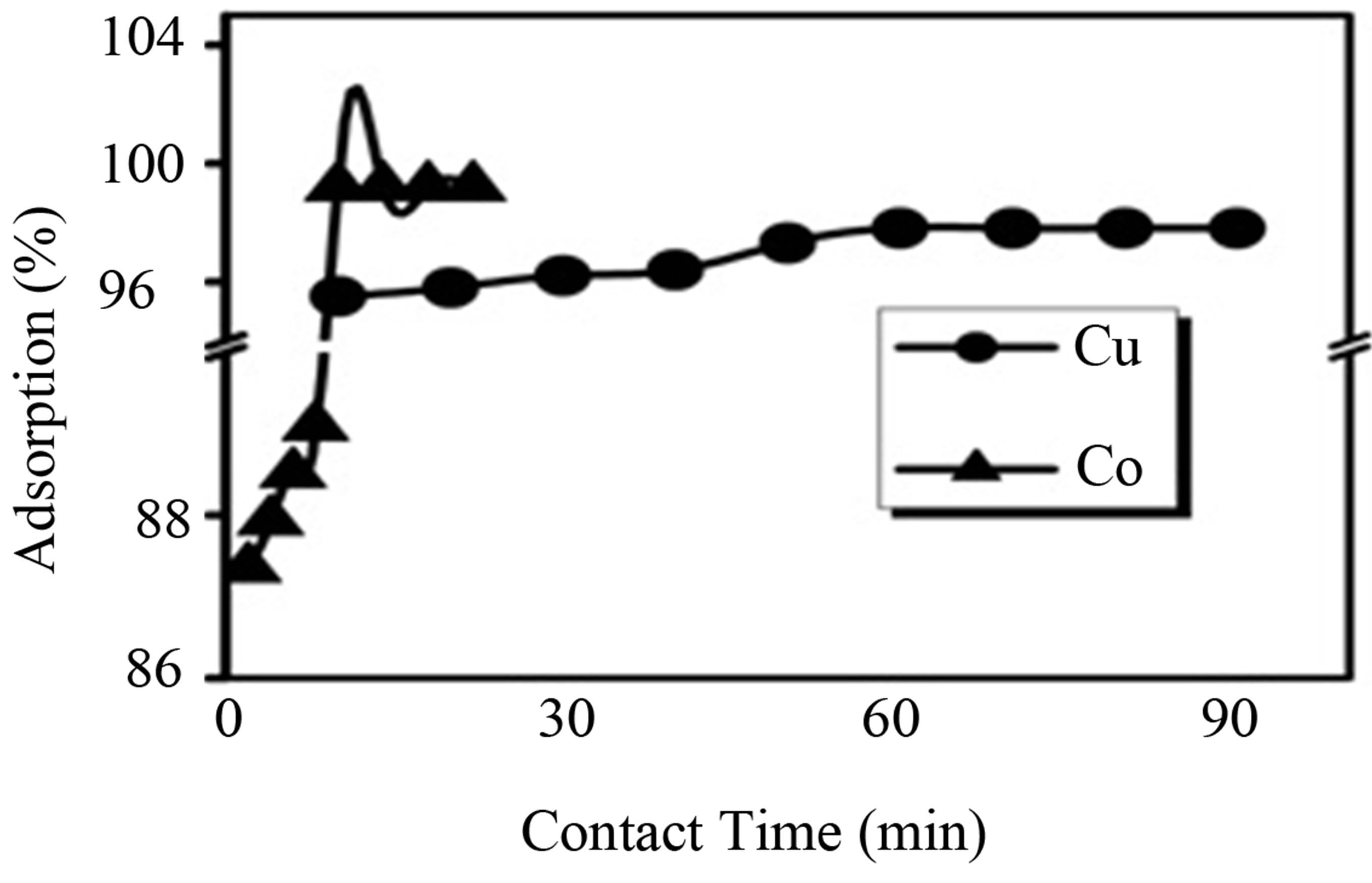
Figure 6. Effect of Contact time in percentage removal of the Cu(II) and Co(II) ions by adsorption on synthetic nano Fe3O4.
3.6. Effect of pH
The pH is one of the important parameter controlling the uptake of Cu(II) and Co(II) ions from aqueous solution by the adsorbent. The adsorption potential of the synthetic nano Fe3O4 was found out at various pH values (1.8 - 6) keeping the system at the following conditions: optimum concentration (50 ppm for both Cu(II) and Co(II) ions), fixed dose of synthetic nano Fe3O4 (1.07 g/L for Cu(II) and 2.57 g/L for Co(II) ions), optimum contact time (60 min for Cu(II) ion and 10 min for Co(II) ions) and temperature 28˚C ± 1˚C. The removal of the Cu(II) and Co(II) ions by synthetic nano Fe3O4 was effective in acidic medium only and the effective pH range was found to be 5.5 for Cu(II) ions and 5.4 for Co(II) ions. This observation reveals that the adsorption process is pH dependent [5,7]. At low pH, because of the higher concentration of the H+ ion and its higher mobility due to smaller size, the H+ ions are preferentially adsorbed compared to the Cu(II) and Co(II) ions, thus lowering the extent of the removal of the metal ions. At optimum pH, the concentration of the H+ ions is lowered which leads to the preferential adsorption of the Cu(II) and Co(II) ions. At higher pH the hydroxide ion concentration is increased and preferentially adsorbed on the adsorbent. Moreover, at higher pH, the Cu(II) and Co (II) ion forms various complex anion, hydroxide complexes, etc., which are retarded by the negatively charged surface of the adsorbent. The plots of the percentage removal of Cu(II) and Co(II) ions with respect to pH change were shown in Figure 7" target="_self"> Figure 7.
3.7. Vibrational Spectral Magnetic Studies (VSM)
It was observed that for the removal of Co(II) ions by synthetic nano Fe3O4 require only 10 minutes for the maximum removal, whereas for Cu(II) ions it requires 60 minutes for the maximum removal. The less time required for Co(II) ion is because Cobalt is Ferro magnetic in nature, hence it was easily attracted by synthetic nano Fe3O4. In the case of Cu(II) ions, which are Dia magnetic in nature, it does not experience magnetic force so it require 60 minutes for the maximum removal. It was further confirmed by Vibrational Spectral Studies and given in Table 1 and the results are shown in Figures 8(a) and (b).

Figure 7. Variation in the percentage removal of the Cu(II) and Co(II) ions by adsorption on synthetic nano Fe3O4.
 (a)
(a)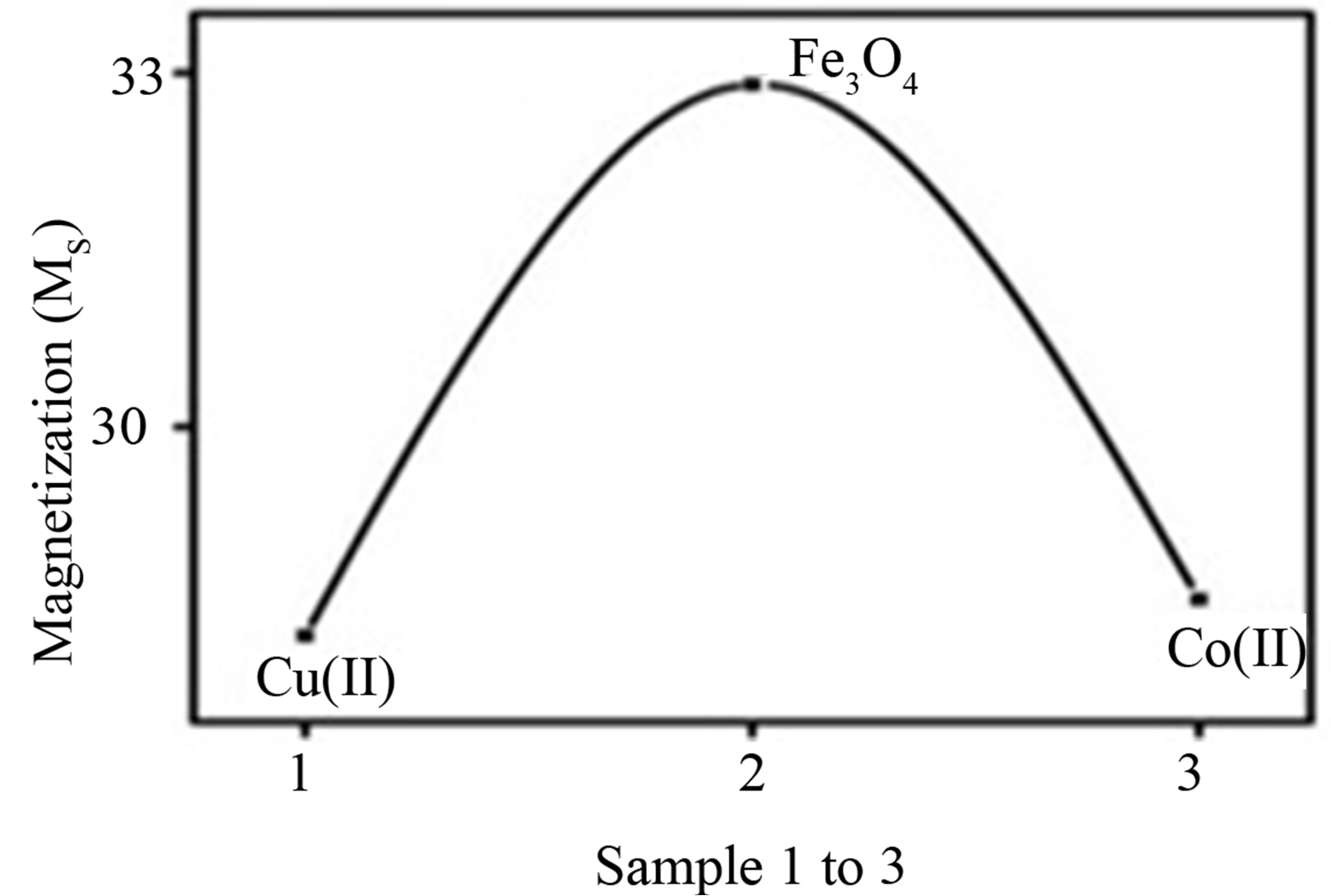 (b)
(b)
Figure 8. (a) and (b) Coercivity Hc and Magnetization Ms nature of synthetic nano Fe3O4 before and after adsorption of the Cu(II) and Co(II) ions.
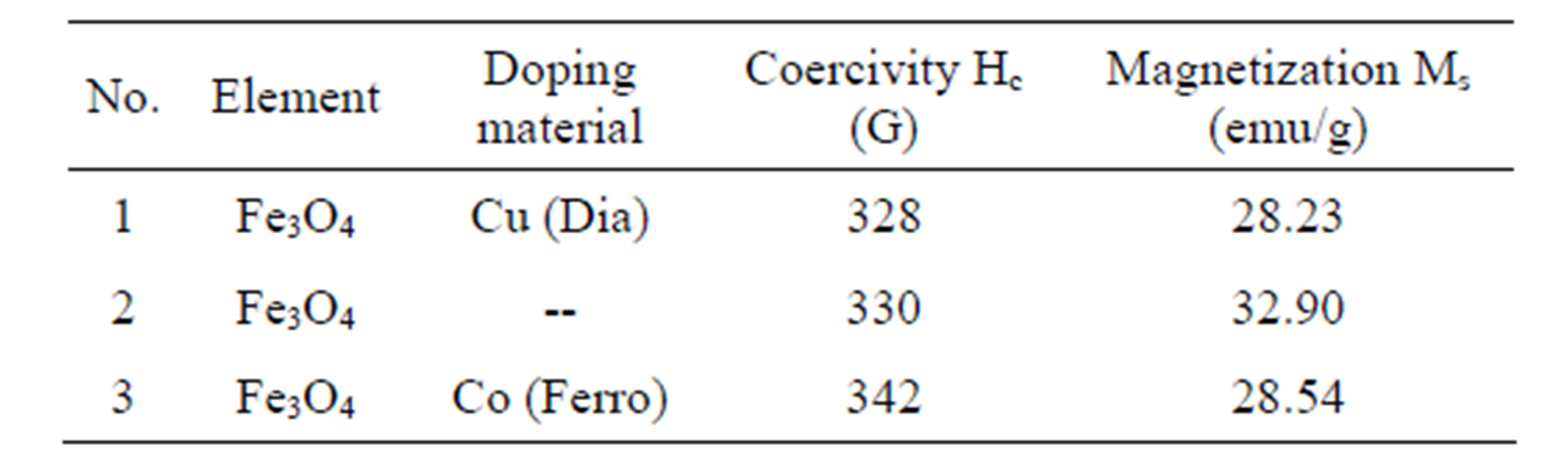
Table 1. Coercivity (Hc) and Magnetization values of synthetic nano Fe3O4 after adsorption of Cu(II) and Co(II) ions.
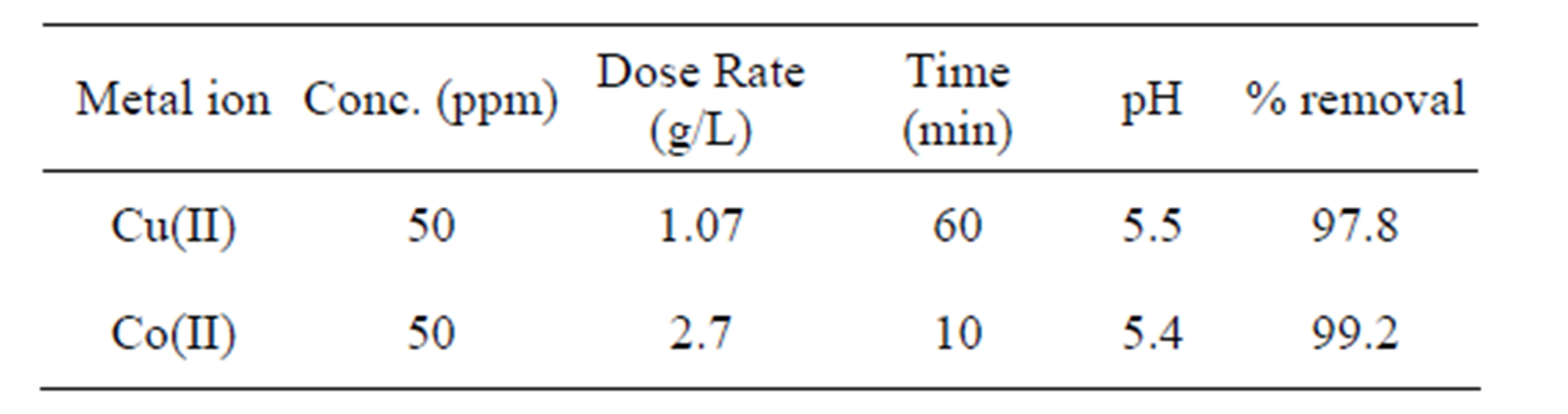
Table 2. Optimum conditions for the removal of Cu(II) and Co(II) ion by using synthetic nano Fe3O4
4. Conclusion
In the present study, the efficiency of the synthetic nano Fe3O4 towards the removal of the Cu(II) and Co(II) ions was examined. Optimum conditions for the removal of Cu(II) and Co(II) ions by using synthetic nano Fe3O4 were given in Table 2. Cobalt is Ferro magnetic in nature, hence it shows optimum contact time of 10 minutes only whereas copper is diamagnetic in nature so it requires the optimum contact time of 60 minutes. The results indicated that the synthetic nano Fe3O4 was found to be more suitable for the removal of Co(II) ions than Cu(II) ions with the minimum contact time of 10min only.
NOTES
Corresponding author.