Production of Iron from Mill Scale Industrial Waste via Hydrogen ()
1. Introduction
In several iron and steel making processes, about 500 kg/ton of solid wastes of different nature are generated; one of these wastes is the mill scale which represents about 2% of steel produced [1]. Mill scale is a very attractive industrial waste due to its richness in iron [about = 72 % Fe) [2].
In the whole world, 13.5 million tons of mill scales are generated annually [3]. Mill scale is suitable for direct recycling to the blast furnace via sintering plant [4]. Approximately, 90% of mill scale is directly recycled within steelmaking industry and small amounts are used for ferroalloys, in cement plants and in the petrochemicals industry [5-8].
El-Hussiny et al. [9] studied the replacement of some amount of Baharia high barite iron ore concentrate by mill scale waste and they indicated that, replacement of some iron ore by mill scale increased the amount of readymade sinter, sinter strength and productivity of the sintering machine and productivity of blast furnace yard.
The reduction of iron oxides via gaseous and solid reductants has already been extensively studied [1].
Maurício et al., studied the kinetics of scale reduction by carbon monoxide in the temperature ranging in 800˚C - 1200˚C and the results indicated that un-reacted shrinking core model with one interface under chemical reaction control fits well with the experimental data at the initial stage of reduction and the activation energy about 80 kJ/mol [1].
Benchiheub et al. [2] found that the best reduction of mill scale was obtained at 1050˚C at 180 min in pure carbon monoxide gas.
It was indicated that with increase in the temperature, the percentage reduction increases with increase in time and the activation energy of reduction depends upon the type of binder [10].
Kawasaki et al. [11] found that varying total pressure of the reduction system by hydrogen and carbon monoxide had no effect on the rate of reduction. Commercial iron powders are classified in three types, reduced iron powder, atomized iron powder, and electrolytic iron powder, depending on the production method and are used in various applications, taking advantage of their respective properties.
Iron powders are used in many different industries for many different applications. Followings are some examples of the iron powder uses: Brazing , Sintered Products, Friction Products, Soft Magnetic Products, Chemicals, Metallurgy, Filtration, Printing, Surface Coating, Welding, Iron Fortification.
Iron powder cores are commonly used to produce high Q inductors and transformers for selective circuits. Iron powder cores used in RF applications are composed of extremely small particles of highly pure carbonyl iron.
This study aims at investigating the reduction kinetic of Egyptian mill scale briquette via hydrogen to produce iron powder.
2. Experimental Work
2.1. Material
The rolling mill scale used in this work was provided by mill of Egyptian iron and steel Co. The sample was submitted to chemical and x-ray analysis was done by Brucker AXS_D8Advnce.
The chemical analyses of mill scale are illustrated in Table 1.
Ray analysis is illustrated in Figure1. From which it is clear that mill scale mainly consists of magnetite, wustite, iron, quartz and hematite.
2.2. Preparation of the Briquetting and Its Physical Properties
The mill scale was grinding in vibrating mill to powder with size less than 75 micrometers. The mill scale powder (10 g) are mixed with 2% molasses and then pressed under different pressure (the pressure range from 75 MPa up to 275 MPa). The briquette subjected to drop number test and crushing strength tests. The drop number indicates how often green briquette can be dropped from a height 46 cm before they show perceptible cracks or crumble. Ten green briquettes are individually dropped on to a steel plate. The number of drops is determined for each briquette. The arithmetical average values of the crumbing behavior of the ten briquettes yield the drop number.

Table 1. the chemical analyses of mill scale.

Figure 1. X-Ray analysis of mill scale sample.
The average crushing strength is done by compressed 10 briquettes between parallel steel plates up to their breaking [12].
2.3. Reduction Process
The reduction of mill scale by hydrogen was done in a thermo balance apparatus. (A schematic diagram of thermo balance apparatus is shown in figure 2 [13]. It consisted of a vertical furnace, electronic balance for monitoring the weight change of reacting sample and temperature controller. The sample was placed in a nickel chrome crucible which was suspended under the electronic balance by Ni-Cr wire. The furnace temperature was raised to the required temperature (650˚C - 950˚C) and maintained constant to ±5˚C. Then samples were placed in hot zone.
The nitrogen flow rate was 0.5 l/min in all the experiments. At initial time air should be removed before each experiment and also after the end of reduction. The weight of the sample was continuously recorded at the end of the run; the samples were withdrawn from the furnace and put in the desiccators.
The percentage of reduction was calculated according to the following equations:
Percent of reduction = 
where:
w0: the initial mass of mill scale sample after removal of moisture.
Wt: mass of sample after each time, t.
Oxygen (mass): indicates the mass of oxygen percent in mill scale in form FeO and Fe2O3.
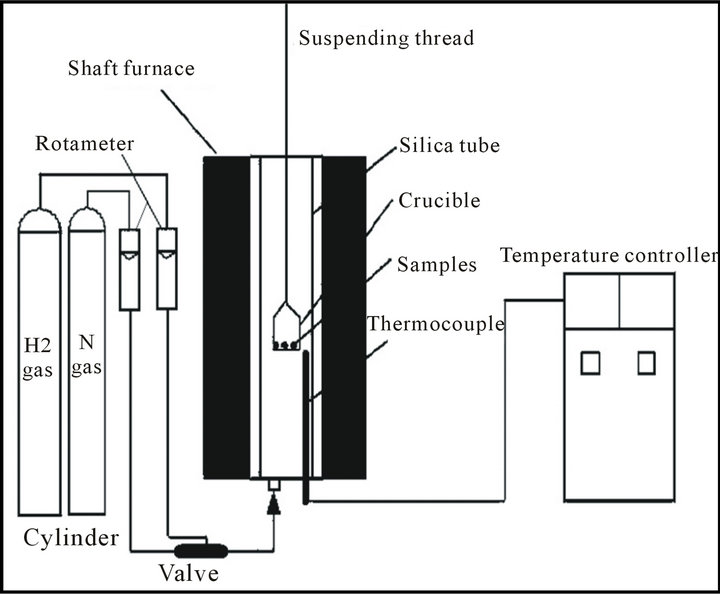
Figure 2. A schematic diagram of thermo balance apparatus (13).
3. Results and Discussion
3.1. Effect of the Pressure Load with Constant Amount of Binding Material on the Quality of the Briquette
Figures 3 and 4 show the relation between the change of pressure load at constant amount of molasses (2%) on the drop number (drop damage resistance) and cold crushing strength of the briquette It is clear that as the pressing pressure load increased both the drop damage resistance and crushing strength increased. This may be due to the fact that increase pressure load increases the compaction of briquette and subsequently the Vander Waals forces increased [14,15], also the increase of briquetting pressure leads to progressive crushing of the macro pores [16].
3.2. Effect of Flow Rate on the Degree of Reduction
Figure 5 illustrated the effect of change hydrogen flow rate on the degree of reduction of mill scale briquette at 900˚C (pressure load 216.85 MPa). From this figure it is clear that the degree of reduction increased as the hydrogen flow rate increased. This may be attributed that the increase of flow rate leads to an increase of number of hydrogen mole in the bulk phase, which in turn leads to the raise of hydrogen adsorption and subsequently the rate of reaction increased [17] or the increase of flow rate increased the gas diffusion across the boundary layer