2. Materials and Methods
2.1. Preparation of Adsorbent
The leaves of Portulaca oleracea were collected from Samno village, Sebha, Libya. They were cleaned from dust and undesirable materials, washed twice with distilled water and dried at 100˚C for two hours. The dried leaves were grounded in a mortar to a very fine powder and sieved through a 125 µm copper sieve. The pH of Pb(II) solution was 4, the doses of Portulaca oleracea leaves in all experiments were 0.05 g and the shaking speed was constant at 300 rpm.
2.2. Reagents
Analytical grade reagents were used in the experimental studies. Lead acetate, xylenol orange, hexamethylene tetra amine and ethylene di amine tetra acetic acid (EDTA) were delivered from MERCK, Germany. Hydrochloric acid, sulfuric acid and sodium hydroxide were supplied from FLUCA, Germany.
2.3. Apparatus
A pH meter, model 3505, Jenway Felsted. Dumow, Essex C.46 SLB, UK was employed for testing the pH of Pb(II) solutions. The batch experiments were carried out using a mechanical shaker, model 501, delivered from Stuart Scientific, UK.
2.4. Preparation of Pb(II) Solutions
A stock solution of 2000 mg/l was prepared by dissolving the 3.14 g of lead acetate in one liter double distilled water.
All working solutions were prepared by diluting the stock solution with double distilled water.
The pH values of the working solutions were adjusted with 0.1 M hydrochloric acid or 0.1 M sodium hydroxide and kept constant at pH 4.
The concentrations of Pb(II) were determined by titration with EDTA in the presence of xylenol orange as indicator.
2.5. Batch Adsorption
In batch experiments, 0.05 g of Portulaca oleracea leaves was added into several 250 ml Erlenmeyer flasks with plastic stopper, each containing 100 ml of Pb(II) solution. The flasks were then shaken at 300 rpm. After shaking, the Pb(II) solutions were filtered and their concentrations were determined.
In order to investigate the kinetics of adsorption, the effect of contact time (10 - 60 minutes) on adsorption capacity of Portulaca oleracea leaves was studied. The isotherm studies were performed by varying the initial Pb(II) concentration from 100 to 1000 mg/l at 295, 303 and 310 K.
The adsorption capacity (qe) of Portulaca oleracea leaves was calculated according to the following equation [7]:
 (1)
(1)
where C0 and Ce are the initial and equilibrium concentration of Pb(II) (mg/l), respectively. V is the volume of Pb(II) solution and W is the weight of Portulaca oleracea leaves.
3. Results and Discussion
3.1. Effect of Contact Time
Figure 1 shows the influence of contact time on adsorption capacity of Pb(II) at 295 K. C0 was 300 mg/l for all cases. The experimental data indicate that the adsorption
capacity increased rapidly at initial contact time of less than 10 minutes and reached to equilibrium at one hour.
3.2. Kinetic Study
In order to suggest the mechanism of adsorption, kinetic models such as first-order, pseudo-second-order and intra-particle diffusion are probably used to test the experimental data.
The first-order rate Lagergren model is [8]:
 (2)
(2)
where qt is the amount of adsorbed Pb(II) on the adsorbent at time t and K1 is the rate constant of the first-order (min−1). K1 and qe can be calculated respectively from the slop and intercept of the plot log(qe − qt) versus t (Figure 2). It is found from Table 1 that the qe value obtained by first-order kinetic model (qcal.) differ from the measured experimentally (qexp.), suggesting the adsorption is not first-order reaction. The value of correlation coefficient (R2) is 0.8755, indicating also that the adsorption is not first-order reaction.
The best fit for the obtained experimental data was carried out by application of pseudo second-order according to the following equation [8]:
 (3)
(3)
where K2 is the rate constant of second-order adsorption (g/mg·min). qe (qcal.) and K2 can be calculated respectively from the slop and the intercept of the plot t/qe versus t (Figure 3). R2 for pseudo second-order was equal to 1 and the qcal. is agree with qexp. (Table 2). Bothe parameters suggest that the adsorption of Pb(II) followed the pseudo second-order kinetic model.
The intra-particle diffusion model is the most common model could be used to confirm the mechanism of adsorption. This model of Weber and Morris can be written as follows [9]:
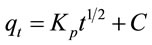 (4)
(4)

Figure 2. The first-order (Lagergren) plot.

Table 1. The first-order (Lagergren) parameters.

Table 2. The pseudo second-order parameters.
where Kp is the intra-particle diffusion rate constant (mg/g min). The linear portion of the plot of qt versus t1/2 is displayed in Figure 4. The adsorption of Pb(II) onto Portulaca oleracea leaves could be interpreted by intra-particle diffusion because of the high value of R2 (0.9993). The plot is not passed through the origin point, suggesting that the pore diffusion may not be the only rate controlling step in the adsorption process [10].
3.3. Adsorption Equilibrium
The adsorption isotherms are the equilibrium relation ships between the concentrations of adsorbent metal and metal in solution at given temperature. The adsorption isotherms of Pb(II) onto Portulaca oleracea leaves are

Figure 4. The intra-particle diffusion plot.
presented in Figure 5.
In the present work, Langmuir, Freundlich and D-R isotherm models were applied to describe the equilibrium data.
The Langmuir isotherm model is based on the hypothesis that adsorption occurs on a homogenous surface by monolayer sorption without interaction between adsorbed molecules [11]. This model is expressed as follows [12]:
 (5)
(5)
where q0 is the adsorption maximum of Pb(II) onto Portulaca oleracea leaves and K (l/mg) is the Langmuir adsorption constant which will be used later to determine the Gibbs free energy .
.
Linear plot of Ce/qe versus Ce as presented in Figure 6 was employed to determine the value of q0 and K. The obtained data as well as R2 values were illustrated in Table 3. The high values of R2 (0.9920 - 0.9950) suggests that the Pb(II) adsorbed by Portulaca oleracea leaves from monolayer coverage on the surface of the adsorbent.
The dimensionless constant separation factor (RL) can be determined according to the following equation [13]:
 (6)
(6)
Accordingly, values of RL at different temperatures were observed to be positive, lying between 0 and 1 (0.154 - 0.833). These results confirmed the favorability of the adsorption isotherm. The diagnostic criterion about the shape of isotherm, is as follows [13]: RL > 1, unfavorable isotherm, RL = 1, linear isotherm, 0 < RL < 1, favorable isotherm and RL = 0, irreversible isotherm.
The Freundlich isotherm is probably used to describe the multilayer sorption of metal ion on a heterogeneous surface of adsorbent according to the following equation [14]:
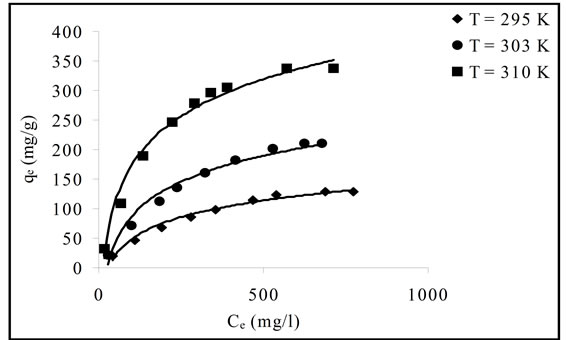
Figure 5. Adsorption isotherm of Pb(II) onto Portulaca oleracea leaves at different temperatures.

Figure 6. Linearlized langmuir isotherm plots (C0, 100 - 1000 mg/l; contact time, 2 hours and adsorbent, 0.5 g).
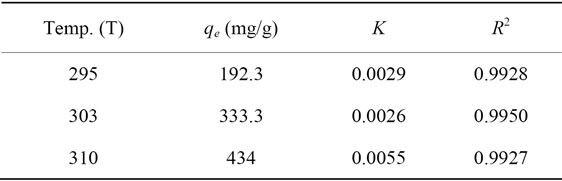
Table 3. Langmuir isotherm parameters for Pb(II) uptake by Portulaca oleracea leaves.
 (7)
(7)
where n and KF are the Freundlich constants which determined respectively from the slop and intercept of the plot of logqe against logCe (Figure 7). The Freundlich isotherm parameters are displayed in Table 4. The R2 values (0.9475 - 0.9722) indicated that the isotherm data is fitted with Freundlich isotherm model but less than Lungmuir isotherm model. From Table 4 it is apparent that the obtained values of n indicate favorable adsorption, as they lie between 1 and 10 [5].
The equilibrium data were also investigated in order to decide the nature of adsorption process. The following equation can be used to calculate the parameters of Dubinin-Radush Kevich (D-R) isotherm model [15]:
 (8)
(8)

Figure 7. Freundlich isotherm plots (C0, 100 - 1000 mg/l; contact time, 2 hours and adsorbent, 0.5 g).

Table 4. Freundlich isotherm parameters for Pb(II) uptake by Portulaca oleracea leaves.
where  is the activity coefficient (mol2·J−2) which can be calculated from the slop of the plot of
is the activity coefficient (mol2·J−2) which can be calculated from the slop of the plot of  against lnqe (Figure 8) and
against lnqe (Figure 8) and  is the Polanyi Potential.
is the Polanyi Potential.
The values of  can be calculated by the use of the following equation [15]:
can be calculated by the use of the following equation [15]:
 (9)
(9)
The mean free energy (E; KJ·mol−1) is determined by the use of  value according to the following equation [15]:
value according to the following equation [15]:
 (10)
(10)
The values of E give an idea about absorption of mechanism wither if physical or chemical. If the values lies between 8 and 16 KJ·mol−1, the adsorption process is classified as chemical adsorption [15].
In the present work, E value was calculated as 0.045 KJ·mol−1. This result suggest that the adsorption process may interpreted by physical mechanism because the value of E < 8 KJ·mol−1.
4. Thermodynamic Study
The thermodynamic parameters such as Gibbs free , enthalpy
, enthalpy and entropy
and entropy 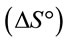 were estimated to evaluate the feasibility and nature of adsorption [16].
were estimated to evaluate the feasibility and nature of adsorption [16].
 for the adsorption of Pb(II) by Portulaca oleracea was calculated using the following equation [16]:
for the adsorption of Pb(II) by Portulaca oleracea was calculated using the following equation [16]:
 (10)
(10)
where R is gas constant (8.314 J·mol−1·K−1).
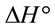 and
and 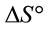 can be determined respectively from the slop and intercept of the plot of 1/T versus lnK (Figure 9) using the following equation [16]:
can be determined respectively from the slop and intercept of the plot of 1/T versus lnK (Figure 9) using the following equation [16]:
 (11)
(11)
The calculated thermodynamic parameters are illustrated in Table 5. The positive values of  suggested the non spontaneous nature of the process.
suggested the non spontaneous nature of the process.  and
and  can be determined respectively from the slop and intercept of the plot of lnK versus 1/T. The positive value of
can be determined respectively from the slop and intercept of the plot of lnK versus 1/T. The positive value of  and
and  indicated that the Pb(II) adsorption process was endothermic in nature and random. It has been reported in literature that the value of
indicated that the Pb(II) adsorption process was endothermic in nature and random. It has been reported in literature that the value of 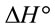 due to chemosorption should be in the range from 40 to 120 KJ·mol−1 [16]. In the present work, the value of
due to chemosorption should be in the range from 40 to 120 KJ·mol−1 [16]. In the present work, the value of  (26.6 KJ·mol−1) confirmed the physisorption process. This result agrees with the results obtained from D-R isotherm model studies.
(26.6 KJ·mol−1) confirmed the physisorption process. This result agrees with the results obtained from D-R isotherm model studies.

Figure 8. D-R isotherm plot (C0, 100 - 1000 mg/l; T, 303 K; contact time, 2 hours and adsorbent, 0.5 g).

Table 5. The thermodynamic parameters of Pb(II) adsorption onto Portulaca oleracea leaves.
5. Conclusion
In this work, the parameters including initial pH, adsorbent dose, particles size of adsorbent and shaking speed are kept constants. The adsorption of Pb(II) onto Portulaca oleracea leaves reached equilibrium after two hours. Batch lead ion uptake capacity tests have shown that the adsorption process can be better described by pseudo second-order model. The intra-particle diffusion may be one of the determining step in the adsorption process. The adsorption data fitted with the Langmuir and Freundlich models. However, Langmuir isotherm displayed a better fitting model than Freundlich isotherm because of the higher correlation coefficient, thus, indicating the adsorption of Pb(II) as a monolayer onto the surface of Portulaca oleracea leaves. The mean free energy value evaluated from D-R isotherm model explained that the adsorption of Pb(II) onto Portulaca oleracea leaves was carried out through physical adsorption mechanism. Thermodynamically, the adsorption process is nonspontaneous, endothermic and random.