n-Dodecylbenzene Sulfonic Acid (DBSA) as a Novel Brønsted Acid Catalyst for the Synthesis of Bis(indolyl)methanes and Bis(4-hydroxycoumarin-3-yl)methanes in Water ()
1. Introduction
Heterocyclic compounds, the highly popular precursor molecules are extensively employed in food, cosmetic and pharmaceutical industries for their broad range of biological activities [1-3]. In pharmaceutical industries coumarin, indole and their derivatives have received great attention due to their therapeutic potential for antibiotic, anti-inflammatory, anti-coagulant, analgesic, antitumor, anti-HIV, anti-apoptotic, cytotoxic, anti-oxidant and insecticidal activities [4-12]. Moreover bis(indolyl) methane derivatives are used as sensors for Cu2+ ions [13]. Bis(indolyl)methanes and bis(4-hydroxycoumarin- 3-yl) methanes are obtained by the condensation of two molecules of indoles or 4-hydroxy coumarin with one molecule of aldehyde or ketone in the presence of a Lewis acid or Brønsted acid catalyst. Literature reports revealed that the Lewis acid catalysts are deactivated or decomposed by the presence of nitrogen in the reactants. Hence, it necessitates having more than stoichiometric amounts of Lewis acid in the reaction mixture [14].
Hitherto, numbers of methods are in use for synthesis of all these biologically active molecules [15-23]. Some of these methods show advantages with respect to each other. Many of these protocols require stoichiometric and expensive catalyst, prolonged reaction time, high temperature, harsh reaction conditions use of organic solvents, tedious and laborious workup procedure, etc. The failure in removal of water formed during the course of reaction leads to low yields of products.
Nowadays, with the awareness towards the environment, researchers are emphasizing on finding out the methods that will fulfill the criteria of environment friendly green chemistry methodologies. In this line, very recently, Thakur et al. [24] used phosphate-impregnated titania catalyst under solvent free condition. Kalita and co-workers [25] used Indion Ina 225 H resin for synthesis of bisindolyl methane. In our recent work, we have used silica supported sodium hydrogen sulfate and Indion 190 resin for the synthesis of bis(4-hydroxycoumarin-3- yl)methanes [26].
Performing the reactions in micellar media instead of organic solvents can alter the reaction rates and the pathways of the reactions. Due to the “local concentration effect” Micelles can concentrate the reactants within their small volumes, stabilize substrates, intermediates or products and orient substrates. A reactant may align at the interface in a “surfactant-like” manner and a water-soluble species will preferentially attack at the polar end while an oil-soluble species at the apolar end of the reactant. Therefore, we may control the reaction rates, mechanism; regioand stereo-chemistry in the presence of micellar media [27-30].
In this context, we are trying to design a protocol that uses micellar media in aqueous condition [31-33]. Present work underlines the effectiveness of micellar media in synthesis of bis(indolyl)methanes and bis(4-hydroxycoumarin-3-yl)methanes (Scheme 1).
2. Results and Discussion
The initial efforts were devoted towards investigation of the best catalyst. We screened various Lewis acids, brønsted acids and surfactants to check the catalyst efficiency for condensation of indole and benzaldehyde. Interestingly, among the catalysts tested, DBSA was found to be the best catalyst with regards to reaction time and yield of the product (Table 1). Further, we extended same protocol for synthesis of bis(4-hydroxycoumarin-3-yl) methanes.
In order to elucidate the role of the DBSA as catalyst, a control reaction was set up using indole (2 mmol) and benzaldehyde (1 mmol) in water in the absence of catalyst. The control reaction offered low amounts of bis (indolyl)methanes after 24 hours (Table 1, Entry 1). However, p-Toluenesulfonic acid (TsOH) and boric acid afforded the products in quantitative yield after 3 and 6 hours, respectively (Table 1, Entries 2, 3). Surfactant in water formed a white turbid reaction mixture indicating that the long chain of the surfactant is necessary for the formation of the colloidal dispersion. Beside this, it was found that the type of surfactant used, influenced both, yield and reaction time. The surfactant like CTAB, SDS and Triton X-100 were found to be less effective for the formation of bis(indolyl)methanes (Table 1, Entries 4 - 7). While in combination of TsOH and sodium dodecyl sulfate (SDS) which formed a colloidal dispersion gave the adduct with significant yield (Table 1, Entry 9). Observations revealed that DBSA is the most efficient surfactant catalyst for this reaction. The enhancement in rate and yield was observed owing to the successful removal


Scheme 1. Synthesis of bis(indolyl)methanes/bis(4-hydroxycoumarin-3-yl)methanes.

Table 1. Optimization of the catalyst for the synthesis of bis (indolyl)methanes in watera.
of water molecule generated during the course of reaction by hydrophobic interior of micelles.
Indeed, concentration of the catalyst also plays vital role in the synthesis of Bis (indolyl) methanes. After varying the concentration of DBSA, we got optimum yield with 10 wt% of catalyst. On further increasing the amount of catalyst, the yield of corresponding product decreased (Table 1). We attributed this to the micelles ability of forming a mask around its own micelle at high concentration which could lead to the inhibition of substrate interactions.
In order to appraise feasibility and scope of this protocol, structurally diverse aldehydes were treated with indole and 4-hydroxycoumarin at the optimized conditions to furnish bis(indolyl)methanes and bis(4-hydroxycoumarin-3-yl)methane derivatives, respectively (Table 2). The products were obtained in good to excellent yields and characterized by 1H NMR, 13C NMR and physical constant. Physical and spectral data of known compounds are in accordance with those reported in the literature [20-26].
3. Conclusion
In conclusion, we have demonstrated that DBSA is an excellent catalyst for the reaction of indoles/4-hydroxy coumarin with aromatic aldehydes to get bis(indolyl) methanes/bis(4-hydroxycoumarin-3-yl)methane derivatives in water. High activity and easy handling makes DBSA as an ideal catalyst for this transformation. The procedure has the advantages of mild reaction conditions, high yields of products, short reaction time, and simple experimental technique, making it a useful and attractive process for the synthesis of bis(indolyl)methane and bis (4-hydroxycoumarin-3-yl)methane derivatives.
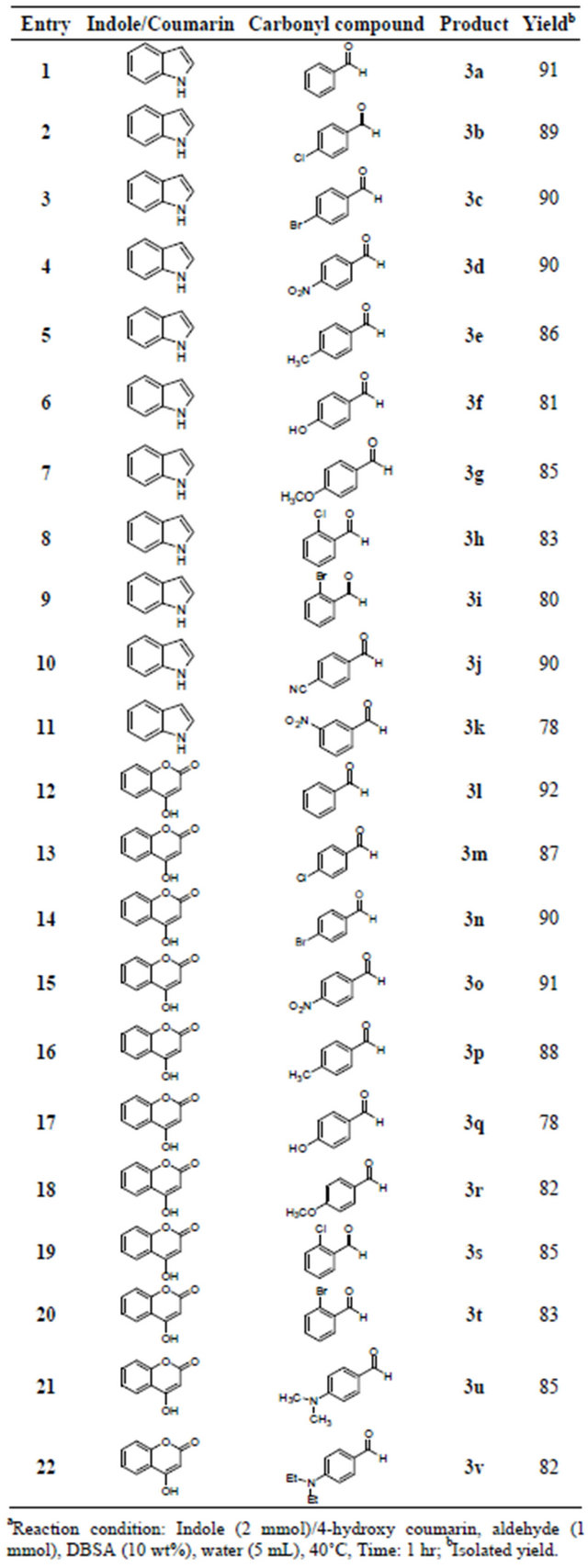
Table 2. Synthesis of bis(indolyl)methanes and bis(4-hydroxycoumarin-3-yl)methanesa.
4. Experimental
4.1. Materials and Methods
All commercial reagents were used as received without purification and all solvents were reagent grade. The reaction was monitored by TLC using 0.25 mm E-Merck silica gel 60 F254 precoated plates, which were visualized with UV light. Melting points were taken in open capillaries. The IR spectra were recorded on a PerkinElmer 257 spectrometer using KBr discs. 1H NMR and 13C NMR spectra were recorded on a VXR-300 MHz instrument using TMS as an internal standard.
4.2. General Procedure
Indole or 4-hydroxycoumarin (2 mmol) and benzaldehyde (106 mg, 1 mmol) were added to 5 mL of water containing 10 wt% of DBSA. The reaction mixture was stirred for 1 h at 40˚C. The progress of the reaction was monitored by TLC. After 1 h, 10 mL of ethyl acetate and 5 mL of water was added to the reaction mixture. The product was extracted in ethyl acetate layer, washed with small quantity of water, dried over sodium sulfate, and evaporated under vacuum to get crude product. The crude product obtained was further purified using column chromatography by eluting with ethyl acetate: hexane (2:8) solvent system.
4.3. Physical and Spectral Data
Compound (3a):
1H NMR: (DMSO-d6): δ 5.86(s, 1H), 6.64 (d, J = 2.26 Hz, 2H),7.00 - 7.57 (m, 13H), 8.45 (brs, 2H, NH, exchangeable with D2O).
13C NMR: (DMSO-d6): δ 31.2, 110.5, 111.0, 118.1, 119.7, 121.0, 123.8, 126.0, 127.4, 128.3, 128.6, 136.8, 144.7 ppm.
IR (KBr): 3390, 3060, 2980, 1610, 1600, 1460, 1110.
Mp: 105˚C (Lit. 104˚C - 105˚C);
Compound (3l):
1H NMR: (DMSO-d6): δ 6.20(s,1H), 6.90 - 7.50 (m, 13H), 11.07 (s, 2H, -OH, exchangeable with D2O).
13C NMR: (DMSO-d6): δ 16.0, 91.0, 105.2, 108.1, 116.0, 117.3, 124.0, 125.4, 126.1, 127.0, 128.7, 130.0, 132.5, 140.0, 163.1, 166.3.
IR (KBr): 3032, 1655, 1609, 754.
Mp: 229˚C (Lit. 228˚C - 230˚C).
NOTES