Relationship between Structure and Thermodynamic Activity of Carbon Black ()
A. M. Amdur,
V. V. Pavlov,
Barnasan Purevsuren,
Lkhamsuren Munkhtuul
.
Institute of Chemistry and Chemical Technology, Mongolian Academy of Sciences, Ulaanbaatar, Mongolia.
Ural State Mining University, Yekaterinburg, Russia.
DOI: 10.4236/aces.2013.31008
PDF
HTML XML
3,411
Downloads
6,235
Views
Citations
Abstract
The role of carbon black is especially important in cokeless metallurgy. Carbon black can be isolated at less hot zones (less than 720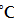 ) in metallurgical ovens according to equation of Buduara: 2CO = C + CO2. The particles of carbon black obtained by the reaction of Buduara are characterizing with complicated open-work structure including globular amorphous parts and graphitized crystalline elements connected by crosspieses with size in nanometric range (0.1μm - 3 μm). The carbon black is characterizing with increased Gibbs’s energy and high kinetical activity because of it’s dispersed and amorphous structure.
) in metallurgical ovens according to equation of Buduara: 2CO = C + CO2. The particles of carbon black obtained by the reaction of Buduara are characterizing with complicated open-work structure including globular amorphous parts and graphitized crystalline elements connected by crosspieses with size in nanometric range (0.1μm - 3 μm). The carbon black is characterizing with increased Gibbs’s energy and high kinetical activity because of it’s dispersed and amorphous structure.
Share and Cite:
A. Amdur, V. Pavlov, B. Purevsuren and L. Munkhtuul, "Relationship between Structure and Thermodynamic Activity of Carbon Black,"
Advances in Chemical Engineering and Science, Vol. 3 No. 1, 2013, pp. 74-77. doi:
10.4236/aces.2013.31008.
Conflicts of Interest
The authors declare no conflicts of interest.
References
|
[1]
|
O. A. Esin and P. V. Geld, “Physical Chemistry of Pyrometallurgical Processes,” 2nd Edition, GNTIL on Ferrous and Nonferrous Metallurgy, Sverdlovsk, 1962, p. 671.
|
|
[2]
|
V. V. Pavlov, “About the Crisis of Kinetic Theory of Liquid and Hardening,” Publisher of USMU (Russian Abbreviation UGGU), Yekaterinburg, 1997, p. 392.
|
|
[3]
|
V. I. Berezkin, “Fullerene as an Nucleus of Carbon Black,” Physics of Solid Body, Vol. 4, No. 3, 2000, pp. 567-572.
|