Determination of Egg Shell Structure and Mineral Composition Using SEM-EDS and Identification the Possibility to Produce Fertilizer ()
1. Introduction
Nowadays, the issue of unusable waste has become increasingly prominent. Eggshells emerge as a potential solution for recycling waste due to their rich content of eggshell carbonate. This compound is efficiently absorbed by plants, playing a vital role in soil alkalinization and enhancing soil structure. Moreover, eggshells contribute significantly to the stimulation of growth processes in young stems, roots, plant photosynthesis, and metabolism [1] [2] . Egg shells consist of complex and complicated substances that are designed to protect not only an embryo, but also provide growing organisms with valuable elements. The main component of egg shells is calcium carbonate, which is 90% - 95% of the solid matter contained in egg shell. In view of the fact that this component is synthesized in bird body, it is reported to be greater absorbed into the soil than the artificial matters [3] [4] . Beyond calcium carbonate, eggshells contain essential minerals like potassium, sodium, magnesium, phosphorus, sulfur, iron, iodine, cobalt, manganese, copper, molybdenum, fluorine, chromium, and zinc, all present in plant-absorbable salt forms [4] [5] . The quality of eggshells is influenced by factors such as age, genetics, nutrition, and environment. As a fertilizer, eggshells prove effective in raising and neutralizing the pH level of excessively acidic soils [6] [7] . The present thesis has been selected as it is considered that there are number of possibilities of using discarded egg shells as valuable ecological product (plant fertilizer) with the view to minimize the impact on environmental pollution.
2. Study Objects and Methods
The calcium carbonate (CaCO3) content in the eggshell was quantified using the reverse titration method, while the eggshell structure and mineral elements were analyzed through SEM-EDS (Scanning Electron Microscopy with Energy Dispersive Spectroscopy) - a cutting-edge methodology. The method of preparation, processing and chemical analysis of egg shells can be seen in the following Diagram 1.
3. Results and Discussion
3.1. Determination of Extracted Alkalinity in Eggshell Samples
The alkalinity in eggshell samples was determined through aqueous extraction. Due to their slow dissolution in water, the alkalinity level increases with prolonged extraction.
Table 1’s initially, the eggshells were washed, sterilized, and ground. Diluted hydrochloric acid solution was added, followed by boiling for 15 - 20 minutes to
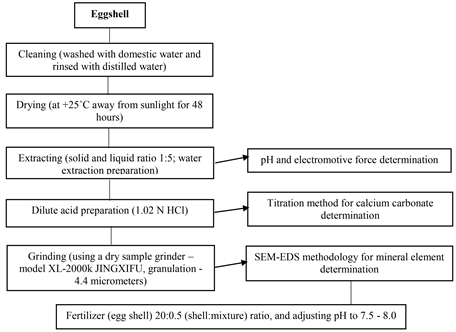
Diagram 1. The order of processing and preparation of eggshells.
![]()
Table 1. Eggshell рН and electromotive force measurement results.
remove carbon dioxide (CO2). After cooling for 2 hours, titration with alkali allowed the calculation of the mass ratio of calcium carbonate (CaCO3) in the eggshells [8] .
3.2. Results of Analyzing the Eggshells Samples through SEM-EDS (Scanning Electron Microscopy with Energy Dispersive Spectroscopy)
Figure 1’s SEM-EDS analysis was conducted on three types of eggshell samples (“Bayan” as “U2”, “Yaitso” Russian as “U3”, and iodized boiled eggshell as “UJ4”). Collected, washed, sterilized, and dried in the laboratory, the eggshells were analyzed without grinding using the SEM-EDS tool. Coded samples were sent for analysis at the Mongolian University of Science and Technology and Nagoya University research center [9] .
As observed in the SEM images magnified from 500 (a) to 1000 (b) times (Figure 2), the eggshell exhibits small cracks, fractures, defects, small pores, and small-scale structures with a uniformly smooth surface and oval-coarse shape. This consistent structure in the light-colored surface is closely linked to the CaCO3 content, aligning with the highest detected Ca percentage of 85.7% according to the EDS peak results.
Figure 3’s EDS results for the “Bayan” (U2) eggshell indicate the highest contents of calcium (85.7%), phosphorus (7.0%), iodine (3.0%), and magnesium (2.7%). This suggests a predominant composition of calcium, magnesium carbonate, and phosphate minerals in this sample. Notably, “Bayan” eggs, belonging to the iodine-enriched category, include iodine as a significant physiological mineral.
Further examination of SEM images magnified at 500 (a) and 1000 (b) times (Figure 4) reveals moderate cracks, fractures, defects, uniform small-sized pores, slightly rough and smooth surfaces, and small-area structures with oval and meander shapes. The consistently distributed structure in the light gray surface correlates with the CaCO3 content, which is measured at 82.8 % in the eggshell.
In Figure 5, the EDS analysis results reveal that the Russian eggshell has the highest content of calcium (82.8%), phosphorus (1.0%), and magnesium (4.5%), indicating a predominant composition of calcium, magnesium carbonate, and phosphate in this sample. Notably, no iodine was detected in the Russian eggshell.
![]()
Figure 1. Content of calcium carbonate in eggshell (%).
![]()
Figure 2. SEM image for the “Bayan” eggshell (U2).
![]()
![]()
Figure 3. EDS results for the “Bayan” eggshell (U2) mineral elements.
![]()
Figure 4. SEM image demonstrating the shell structure for the “Yaitso” russian eggshell (U3).
Moving to Figure 6, the SEM image of the iodized boiled eggshell, magnified at 500 (a), and 1000 (b) times, displays slight cracks, fractures, uniform small pores, and a distinct structural change. The boiling process is evident in the separation of hollow parts and the presence of oval and round-shaped particles.
![]()
![]()
Figure 5. EDS results for the “Yaitso” russian eggshell (U3).
![]()
Figure 6. SEM image for the iodized boiled egg (UJ4).
Figure 7, the EDS results for the iodized boiled eggshell, show the highest peaks for calcium (72.6%), phosphorus (18.1%), magnesium (3.7%), and iodine (2.6%). This indicates a predominant composition of calcium, phosphorus, and magnesium in this sample. Importantly, the enriched iodine content is retained even after the boiling process. The table below summarizes the mineral element content of the samples (Bayan, Yaitso and Iodized eggshells).
![]()
![]()
Figure 7. EDS results for the iodized boiled eggshell (UJ4) mineral elements.
Table 2 summarizes the mineral content in various eggshells, revealing that calcium ranges from 72.6% to 85.7%, magnesium from 2.7% to 4.7%, phosphorus from 7.0% to 18.1%, sulfur from 0.5% to 2.0%, potassium from 0.6% to 0.4%, and iodine from 3% to 2.6%. The EDS peak results suggest that the mineral structure of “Bayan” and Russian eggshells is primarily calcium carbonate, while the iodized boiled eggshell consists mainly of calcium carbonate and phosphate. The higher magnesium content in imported Russian eggshells (Yaitso - 4.5%) is significant for the normal functioning of plant enzymes and serves as a valuable raw material for further fertilizers. However, the silicon content in Russian eggshells was relatively low (0.3% - 0.6%), potentially impacting the plant root system. Given the importance of silicon for photosynthesis and root system activity, the use of iodized eggshells is recommended.
Phosphorus, ranking second after calcium in mineral element content among the researched eggshells, is most abundant in iodized boiled eggshells (18.1%). Phosphorus supports the growth of roots, flowers, and fruits. Iodized boiled eggshells also show higher sulfur content, which is crucial for addressing nutrient deficiencies when plant leaves turn yellow [10] . Potassium content in the samples ranges from 0.4% to 0.9%. Potassium’s presence in the plant’s vegetative organs enhances growth, especially when used alongside organic fertilizers.
The calcium content in all three eggshell samples ranges from 72.6% to 87.7%. Calcium plays a vital role in plant growth, participating in cell division, elongation, and hydrogen ion detoxification. Notably, calcium content is 10.2% to 15.1% higher in Bayan and Yaitso eggshells compared to iodized eggshells.
After washing, drying, and sterilizing, the eggshell samples were ground with a dry sample grinder, creating three mixtures (eggshell, tangerine, and lemon peel). The relationship between water extraction duration and pH was examined over 24 - 96 hours, with Mixture 1, exhibiting suitable pH for plants, ultimately chosen for further testing.
Figure 8 illustrates the pH changes in the three mixtures over 24, 48, 72, and 96 hours. Mixture 1 displayed a pH range suitable for plant growth
Examining the SEM image (Figure 9), the dry sample’s small particles vary in size and mostly exhibit an oval-oblong shape. Hard calcium carbonate particles
![]()
Table 2. Content of mineral elements in the samples (%).
![]()
Figure 8. Fertilizer mixture pH and time ratio.
![]()
Figure 9. SEM-EDS results of fertilizer mixture mineral elements.
are distinct, and mechanochemical processing resulted in carbonate decomposition, enhancing surface activity and absorption ability. The small particle size is 4.4 μm. EDS results indicate the highest peak for Ca (97.7%), while the amounts of Mg, Si, P, S, and K decreased, possibly due to mineral decomposition. The testing of the eggshell fertilizer’s effects on indoor flower growth has been initiated.
4. Conclusions
The calcareous shell of eggshells is predominantly composed of calcium carbonate (CaCO3), with variations in quality and content influenced by hen breed and feed. The determined content of CaCO3 in the three samples (U2 - 92%, U3 - 91%, UJ4 - 91.5%) aligns with previous studies and press reviews (90% - 95%).
SEM-EDS analysis revealed a diverse range of macro and micro elements in eggshells (Ca 72.6% - 85.7%, Mg 2.7% - 3.7%, P 7.0% - 18.1%, S 0.5% - 2.0%, Si 0.3% - 0.6%, K 0.4% - 0.9%, I 2.6% - 3.0%). This suggests potential applications for eggshells as raw materials in medicine, food, and plant fertilizers.
Extracting fertilizer without additional or chemical treatment involved grinding eggshells to 4.4 μm, preparing a 20:0.5 (shell: mixture) ratio, and adjusting pH to 7.5 - 8.0.