1. Introduction
The human, social and economic costs of hunger, food insecurity and malnutrition for society as a whole are considerable in terms of lost productivity, health, well-being, reduced ability to learn and limitation of human potential. If current conditions persist, the Sustainable Development Goal of ending hunger by 2030 will not be achieved. Indeed, unless we make our food systems more nutrition-sensitive, large segments of the population, especially in sub-Saharan Africa and South Asia, will remain undernourished in 2030 and even 2050 [1] . However, most of these countries benefit from favorable eco-geographical conditions for the development of a rich and varied flora including a significant potential in food, oilseed, aromatic and medicinal plants, a large part of which is endemic. This endemic character gives them the advantage of producing new natural assets with added value; this is the case of plants with edible fruits whose fruits are highly appreciated for their flavor. The exploitation of this floristic richness is still unconventional, due to still embryonic physicochemical data [2] [3] . Among this richness is the species Pseudospondias microcarpa (A. Rich) Engl., a plant of the Anacardiaceae family, which is generally found in tropical and subequatorial climates. It exists in nature in two forms: the variety with red fruits and the variety with blue-blackish fruits when ripe. Its fruits are edible and highly appreciated by populations as well as chimpanzees for their sweet and tangy taste. Many studies confirm some of the medicinal properties attributed to this plant, including antisplasmodial, antimicrobial, antioxidant, analgesic, anticonvulsant, antidepressant, anxiolytic properties. Some phytochemical studies of this plant have revealed the presence of several major phytochemical families such as triterpenes, polyphenols, flavonoids, alkaloids, tannins, steroids, saponosides, cardiac glycosides and sugars, as well as certain fruit nutrients of the blue-blackish variety [4] [5] [6] [7] .
In Congo, vegetable oil analysis of the fruit seeds of Pseudospondias microcarpa (A. Rich) Engl. delivered several compounds including twelve major compounds, namely oleic acid, palmitic acid, stearic acid, β-Sitesterol, l'α, γ and δ-tocopherol [8] . That of the essential oils of the pulps, stones, shells and seeds of these fruits made it possible to identify 67 compounds, the most important of which are vaccenic acid, ascorbic acid, 2,6-dihexadecanoate and α-terpineol [9] . To our knowledge, no study reports the nutritional value of the fruits and fruit juices of this plant. However, the valorization of these products requires a precise knowledge and justifies this work of determination of the chemical composition, in order to highlight a possible specificity.
2. Material and Methods
1) Collection of plant material
About 50 kg of ripe fruits of Pseudospondias microcarpa (A. Rich) Engl. were harvested along the Kouyou River in Owando, a town located in the central Cuvette department in northern Congo. The botanical identification of the fruit was carried out at the Center for the Study of Plant Resources (CERVE in french). The species was registered under number 8957 on 7/08/1961.
2) Physical characteristics of harvested ripe fruit
The weights of fruits, pericarp, pulp and seeds were measured using an electronic scale and expressed in grams (g). The peel/pulp ratio was determined by taking the ratio between the peel weight and the pulp weight. The fruit color was recorded using the LEROUX color chart. The fruit length and width were measured using a digital caliper and expressed in millimeters (mm) [10] .
3) Determination of dry matter and water content
The dry matter content is evaluated according to the AOC method [11] . This method corresponds to the loss in mass after complete drying at 105˚C, for approximately 2 to 4 hours, in an isothermal oven until a constant mass is obtained. The dry matter content is calculated by formula (1).
(1)
or
DM: Dry matter (%);
Mc: Mass of the empty capsule (g);
Mf: Final mass (g) of the dish containing the sample after staving;
Mi: Initial mass (g) of the capsule containing the sample before staving.
The humidity (or water content) denoted H, is determined from formula (2).
(2)
4) Obtaining fruit juice
The fruit juice was obtained by crushing ripe fruit. 1.5 kg of fresh fruit were manually pressed to collect the pure juice without adding water.
5) Determination of nutrients and phytonutrients
a) Preparation of extracts
A few grams of pulped fresh fruit had been macerated only in ethanol. At the same time, 1 g of dry pulp was subjected to extraction with different solvents, in ascending order of polarity, namely: hexane, chloroform, ethylacetate and water.
b) Screening of chemical families of extracts
The screening of the chemical families was carried out by thin layer chromatography (TLC) and by tube reactions according to the methods described in the literature. The operating conditions of the chemical families sought by TLC are listed in Table 1. The saponosides were determined by the mouse index and the reducing sugars by the Fehling test [12] [13] .
c) Physico-chemical and biochemical analyzes of raw fruit juice
The Nutritional potential of raw fruit juice was determined according to standards and methods described in the literature. The operating conditions for measuring of pH, turbidity, density, titratable acidity and ˚Brix; and determination of water contents, dry matter, ash, total carotenoids, total polyphenols, total flavonoids, tannins, total sugars and dietary fibers as well as the assessment of antiradicaire activity are inscribed in Table 2.
![]()
Table 1. Operating conditions for chemical screening by TLC.
![]()
Table 2. operating conditions for physicochemical and biochemical analyzes.
Calcium, iron, copper and zinc were determined using an inductive coupling optical emission spectrophotometer (ICP-OES) according to the AOC method, n˚ 984.27 [14] . Potassium was determined using an atomic flame absorption spectrophotometer (AAS) according to the AOC method, n˚ 975.03 [15] .
3. Results and Discussion
1) Physical characteristics of Pseudospondias microcarpa (A. Rich) Engl. fruits
The weight of harvested Pseudospondias microcarpa (A. Rich) Engl. varies from 2 to 8 g with a constant pulp/mesocarp-kernel ratio. The size of these fruits varies from 1 to 2 cm in central diameter and 1 to 2.5 cm in length. The color chart allows us to say that the fruit ripening color ranges from yellow to red (final stage). The Observation of the fruits of this plant with the naked eye is reminiscent of those of Spondias mombin L. and Prunus laurocerasus L. in terms of morphology (shape, size and weight) [24] [25] [26] [27] .
2) Water, dry matter and fruit juice content
The water and dry matter contents of this fruit are respectively 69.99% and 31.11%. These results show that the weight of the fruit is dominated by water; which is consistent with the usual content of fleshy fruits described in the literature.
In addition, out of 1500 g of the fruits, 350 mL of raw juice was obtained; i.e. a content of 23.33% of the pure juice in the fruit without adding water. This content makes it possible to classify these fruits among citrus fruits or melons [28] .
3) Highlighting nutrients and phytonutrients in fruits
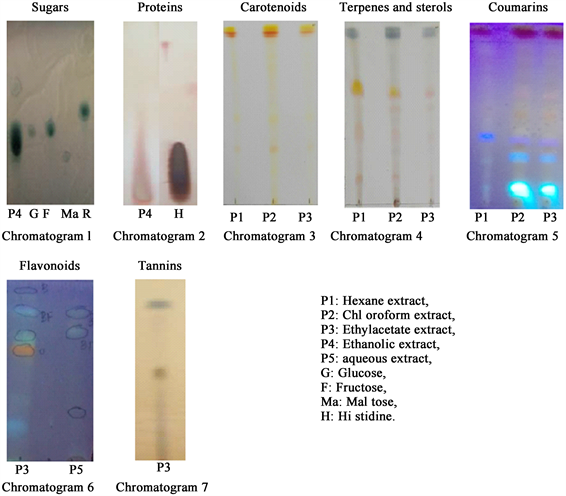
Five extracts were obtained from the fruit pulp. These extracts made it possible to observe by TLC the spots of green, brown, blue (fluorescent), green, yellow, orange, red and gray color characteristic of the presence of two nutrients (sugars and amino acids) and six phytonutrients (carotenoids, coumarins, favonoids, tannins, terpenes and sterols) (Chromatograms 1 to 7). The use of special reagents and elution systems as well as control compounds made it possible to differentiate them according to the results described in the literature.
Chromatograms 1 and 2 show that this fruit could contain energizing compounds namely glucose, fructose, maltose and histidine or their derivatives. The presence of glucose, fructose and maltose in the ethanolic extract confirms the abundance of reducing sugars observed with the Felling test. We also note in this fruit a diversified phytonutrient profile (Chromatograms 3 to 7).
4) Physicochemical and biochemical characteristics of raw fruit juice
Table 3 groups together the physicochemical and biochemical characteristics of the raw fruit juice of Pseudospondias microcarpa (A. Rich) Engl. with those of two varieties of Anacardiaceae taken as references. The juice showed contents of 3.771% ± 0.013% and 0.6047 ± 0.0629 mg/100g respectively for dry matter and ash. These values show that the weight of the juice is more than 95% represented by water. It can be a source of water, an essential element for the functioning of the body. This juice is denser than water (ρjuice = 1.255 g/cm3), very cloudy and colored (turbidity of 1019 NTU); which reflects a high rate of dissolved
![]()
Table 3. Physico-chemical and biochemical quality of Pseudospondias microcarpa fruit juice.
organic matter such as carotenoids. It is moderately acidic (pH 3.28); an acidity approaching that of its Anacardiaceae counterparts taken as examples [29] [30] . The value of ˚BRIX (12.533%) in the juice is high; which also reflects the high sugar content (8.700 ± 0.141 g/100g juice). This fruit is a more energizing nutrient than that of Irvingia gabonensis whose ˚BRIX is 10.00%.
In this juice, polyphenols (134.8500 ± 0.0023 mg gallic acid/100g) are the most abundant phytonutrients than carotenoids (5.100 ± 0.010 mg carotene/100g). These phytonutrients are known for their antioxidant power and their provitamin A property. The presence of these phytonutrients in this juice proves interesting in the prevention of cataracts, age-related macular degeneration, cardiovascular diseases and various cancers such as lung cancer and prostate cancer [31] .
Five mineral elements have been determined and quantified in this juice, namely: copper, iron, calcium, zinc and potassium. Calcium (3570.00 ± 2.05 mg/100g) and potassium (4576.00 ± 9.49 mg/100g) contents are high in the juice because these nutrients are macronutrients. This trend is the same observed with mango juice containing calcium (150.00 mg/100g) and potassium (598.00 mg/100g). The presence of calcium in this juice is therefore important because it gives this juice a power of growth and hardness of bones and teeth. The three other elements identified as, for example, iron and copper are essential for the synthesis and functioning of hemoglobin, the functional role of which is to ensure the transport of respiratory gases. The deficiency of these mineral elements in the body leads to decalcification, rickets, tetany, muscle spasm, fatigue, anemia, growth retardation, pallor, reduced resistance to disease [32] - [37] .
The juice of this fruit has an important antioxidant power characterized by the low percentage of inhibition (3.935% ± 1.015%). This significant activity could be due to the presence of phytonutrients such as phenolic compounds and carotenoids in the juice. This result confirms the use of this fruit for food purposes.
4. Conclusion
The physicochemical and biochemical characterization of Pseudospondias microcarpa (A. Rich) Engl. fruits made it possible to identify functional nutrients and phytonutrients and to quantify carotenoids (5.100 ± 0.010 mg carotene/100g) and polyphenols (134.8500 ± 0.0023 mg gallic acid/100g) as well as five essential mineral elements namely copper, iron, calcium, zinc and potassium with the respective contents 5.100 ± 0.010 mg, 3.02 ± 0.03 mg, 3570.00 ± 2.05 mg, 2.57 ± 0.08 mg and 4576.00 ± 9.49 mg. In addition, these compounds exhibited good antioxidant activity. The presence of these nutrients and phytonutrients militates to give it a status of health food for new technological routes.
Acknowledgements
Our thanks go to Mr. Nicola Ayessou from the University of Dakar (Senegal) for agreeing to carry out the analyses of this work in his laboratory.