1. Introduction
Polyatomic structures are the result of interactions between the valence electrons of atoms that form chemical bonds [1] - [6] .
In a Lewis diagram, atoms are represented by their chemical symbol, surrounded by one or more dots representing valence electrons. Covalent bonds are represented by a line connecting two atoms, with each atom sharing a pair of electrons. Non-bonding electrons, also known as lone electrons, are represented by pairs of electrons located on a single atom.
The use of Lewis diagrams is useful for understanding the molecular geometry, chemical properties and reactivity of molecules [7] - [12] .
The sequence of the eight rules of the systematic method for constructing Lewis representations of buildings are:
Step 1: Determine the total number of valence electrons (nE.V) in the molecule, which is equal to the sum of the number of valence electrons nE V in each atom of the molecule minus the building block charge according to the following relationship:
nE.V(building) =
– charge of the building (Relationship 1)
Step 2: Determine the number of binding and free doublets in the molecule (nD):
nD(building) = 1/2 total number of valence electrons of the building nE V (Relationship 2)
If the number of doublets is not integer but half-integer, we are dealing with a radical (single electron).
Step 3: Determine the number of anti-binding doublets in the molecule (nD.A.L):
nD.A.L(building) =
– charge of the building (Relationship 3)
Step 4: Determine the number of binding doublets in the molecule (nD.L):
nD.L(building) = nD(building) – nD.A.L(building) (Relationship 4)
Step 5: Determine the geometry according to Gillespie, which tells us about the spatial geometry of the species:
The geometry of a molecule around a given atom will be that for which the repulsions between the doublets are minimal.
Gillespie gave the rules for the orientation of bonds around the central atom of a molecule.
Gillespie postulate:
- All pairs of electrons (binding or non-binding) are at the same distance from the nucleus.
- These pairs are assumed to be located on the surface of a sphere with the nucleus at its center.
- These doublets repel each other, and are furthest apart to minimize electron repulsions.
- The V.S.E.P.R formulation is expressed as follows: AXnEn': (Relationship 5).
A: Central atom.
X: atoms or molecules covalently bonded to atom A and n their number.
E: free doublets or single electrons and n' their number on the valence layer of atom A.
Gillespie considers that multiple bonds behave like single bonds, to establish the V.S.E.P.R. formulation, it is necessary to establish the Lewis representation.
Step 6: Position any formal load(s).
The formal charge carried by an atom corresponds to the difference in valence electrons between the isolated atom and the atom when the edifice is formed, all bonds being considered purely covalent.
The sum of all formal charges is equal to the charge of the species.
C.F(atom) = nE.V(atom in isolated state) – nE.V(atom linked to the structure) (Relationship 6)
Step 7: Build the structure by positioning the doublets (binding—by single, double or triple bond—or non-binding), respecting the octet rule as far as possible and placing any single electron.
Step 8: Determine the oxidation number of each atom in the structure.
The oxidation number of an atom corresponds to the difference in valence electrons between the isolated atom and the atom when the structure is formed, all bonds being considered purely ionic.
The sum of the oxidation numbers is equal to the formal charge of the species
d.o(atom) = nE.V(atom in isolated state) – nE.V(atom bound to the structure) (Relationship 7)
2. Constructing Lewis Representations of Buildings Using the Systematic Method
In the following, we present the construction of Lewis representations according to the systematic method for some of the following molecules:
;
;
; PCl5; SO2; H2SO3;
; Fe3O4; Pb3O4; H2O2.
2.1. The Building
Step 1:
Applying relation 1, we find: nE V(
) = nE.V(N) + 4nE.V(H) – 1
Z(N) = 7 from where [He] 2s2 2p3 → nE.V(N) = 5
and Z(H) = 1 from where 1s1 → nE.V(H) = 1
nE.V(
) = 5 + 4 × 1 – 1 = 8
Step 2:
Applying relation 2, we find:
nD(
) = 1/2 × nE V(
) = 8/2 = 4 binding and non-binding doublets
Step 3:
Applying relation 3, we find: nD.A.L(
) = nD.N.L(N) + 4nD.N.L(H) – 1
Z(N) = 7 from where [He] 2s2 2p3 → nD.N.L(N) = 1
and Z(H) = 1 from where nD.N.L(H) = 0
nD.A.L(
) = 1 + 4 × 0 – 1 = 0 free doublets
Step 4:
Applying relation 4, we find:
nD.L(
) = nD(
) – nD.A.L(
)
so nD.L(
) = 4 – 0 = 4 Bonding doublets
Step 5:
The V.S.E.P.R formulation is expressed as follows: AXnEn':
N: central atom
Four hydrogen atoms covalently bonded to the nitrogen atom and n = 4 their number.
E: free doublets and n' = 0, zero their number on the valence layer of the nitrogen atom.
The type of molecule according to Gillespie is NH4 and the basic geometry tetrahedral, as shown in the diagram opposite.
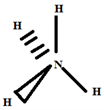
Step 6:
Applying relation 6, we find:
C.F(N) = nE.V(N in isolated state) – nE.V(N linked to the building) = 5 – 4 = + 1
C.F(H) = nE.V(H in the isolated state) – nE.V(H bound to the structure) = 1 – 1 = 0 no charge for the four hydrogens
Step 7:
Build the structure using the systematic method:
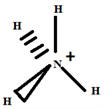
2.2. The Species
Step 1:
nE.V(
) = nE.V(N) + 2nE.V(H) – (–1)
Z(N) = 7 from where nE.V(N) = 5 and Z(H) = 1 from where nE.V(H) = 1
nE.V(
) = 5 + 2 × 1 + 1 = 8
Step 2:
nD(
) = 1/2 × nE.V(
) = 8/2 = 4 binding and non-binding doublets.
Step 3:
nD.A.L(
) = nD.N.L(N) + 2nD.N.L(H) – (–1)
Z(N) = 7 from where nD.N.L(N) = 1 and Z(H) = 1 from where nD.N.L(H) = 0
nD.A.L(
) = 1 + 2 × 0 + 1 = 2 free doublets.
Step 4:
nD.L(
) = nD(
) – nD.A.L(
)
so nD.L(
) = 4 – 2 = 2 Bonding doublets.
Step 5:
The V.S.E.P.R formulation is expressed as follows: AXnEn':
N: central atom.
H: two atoms covalently bonded to the N atom and n = 2 is the number of covalent bonds that hydrogen can form with nitrogen.
E: two free doublets on the valence layer of the N atom.
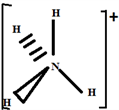
So the Gillespie type of molecule is NH2E2 and the basic geometry tetrahedral, as shown in the diagram opposite.
Step 6:
C.F(N) = nE.V(N in isolated state) – nE.V(N linked to the building) = 5 – 6 = –1
C.F(H) = nE.V(H in the isolated state) – nE.V(H bound to the structure) = 1 – 1 = 0 no charge for the two hydrogens.
Step 7:
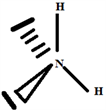
Build the structure using the systematic method:
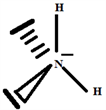
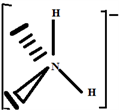
2.3. The Species
Step 1:
nE.V(
) = nE.V(N) + 2nE.V(O) – (–1)
Z(N) = 7 from where nE.V(N) = 5 and Z(O) = 8 from where [He] 2s2 2p4 → nE.V (O) = 6
nE.V(
) = 5 + 2 × 6 + 1 = 18
Step 2:
nD(
) = 1/2 × nE.V(
) = 18/2 = 9 binding and non-binding doublets.
Step 3:
nD.A.L(
) = nD.N.L(N) + 2nD.N.L(O) – (–1)
Z(N) = 7 from where nD.N.L(N) = 1 and Z(O) = 8 from where nD.N.L(O) = 2
nD.A.L(
) = 1 + 2 × 2 + 1 = 6 free doublets.
Step 4:
nD.L(
) = nD(
) – nD.A.L(
)
so nD.L(
) = 9 – 6 = 3 bonding doublets.
Step 5:
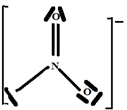
The V.S.E.P.R formulation is expressed as follows: AXnEn':
N: central atom.
O: two atoms covalently bonded to the N atom and n = 2 is the number of covalent bonds oxygen can form with nitrogen.
E: one free doublet on the valence layer of the N atom.
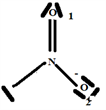
So the Gillespie type of molecule is NO2E1 and the basic geometry triangular planar as shown in the diagram opposite.
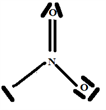
Step 6:
C.F(N) = nE.V(N in isolated state) – nE.V(N linked to the building) = 5 – 5 = O no formal charge on N.
C.F(O1) = nE.V(O1 in the isolated state) – nE.V(O1 bound to the structure) = 6 – 6 = 0 no formal charge on O1.
C.F(O2) = nE.V(O2 in isolated state) – nE.V(O2 bound to the structure) = 6 – 7 = –1.
Step 7:
Build the structure using the systematic method:
2.4. The Species PCl5
Step 1:
nE V(PCl5) = nE.V(P) + 5nE.V(Cl) – 0
Z(P) = 15 from where [Ne] 2s2 2p3 → nE.V(P) = 5
and Z(Cl) = 17 from where [Ne] 2s2 2p5 → nE.V(Cl) = 7
nE V(PCl5) = 5 + 5 × 7 – 0 = 40
Step 2:
nD(PCl5) = 1/2 × nE V(PCl5) = 40/2 = 20 binding and non-binding doublets.
Step 3:
nD.A.L(PCl5) = nD.N.L(P) + 5nD.N.L(Cl) – 0
Z(P) = 15 from where nD.N.L(P) = 1 and Z(Cl) = 17 from where nD.N.L(Cl) = 3
nD.A.L(PCl5) = 1 + 5 × 3 – 0 = 16 free doublets.
Step 4:
nD.L(PCl5) = nD(PCl5) – nD.A.L(PCl5)
So nD.L(PCl5) = 20 − 16 = 4 Binding doublets.
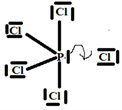
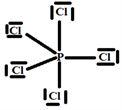
Step 5:
The V.S.E.P.R formulation is expressed as follows: AXnEn':
P: central atom.
Cl: four atoms covalently bonded to the P atom and n = 4 is the number of covalent bonds that chlorine can form with phosphorus.
E: one free doublet on the valence layer of atom P.
So the Gillespie type of molecule is PCl4E and the basic geometry trigonal bipyramid, as shown in the diagram opposite.
Step 6:
C.F(P) = nE.V(P in isolated state) – nE.V(P linked to the building) = 5 − 5 = 0 no formal charge on P.
C.F(Cl) = nE.V(Cl in isolated state) – nE.V(Cl bound to the structure) = 7 − 7 = 0 no formal charge on the five Cl atoms.
Step 7:
Build the structure using the systematic method:
2.5. The Species SO2
Step 1:
nE.V(SO2) = nE.V(S) + 2nE.V(O) − 0
Z(S) = 16 hence [Ne] 2s2 2p4 → nE.V(S) = 6 and Z(O) = 8 hence nE.V(O) = 6
nE.V(SO2) = 6 + 2 × 6 − 0 = 18
Step 2:
nD(SO2) = 1/2 × nE.V(SO2) = 18/2 = 9 binding and non-binding doublets.
Step 3:
nD.A.L(SO2) = nD.N.L(S) + 2nD.N.L(O) − 0
Z(S) = 16 hence nD.N.L(S) = 2 and Z(O) = 8 hence nD.N.L(O) = 2
nD.A.L(SO2) = 2 + 2 × 2 − 0 = 6 free doublets.
Step 4:
nD.L(SO2) = nD(SO2) – nD.A.L(SO2)
so nD.L(SO2) = 9 − 6 = 3 Binding doublets.
Step 5:
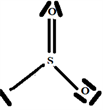
The V.S.E.P.R formulation is expressed as follows: AXnEn':
S: central atom.
O: two atoms covalently bonded to the S atom and n = 2 is the number of covalent bonds oxygen can form with sulfur.
E: one free doublet on the valence layer of atom S.
So the Gillespie type of molecule is SO2E and the basic geometry triangular planar, as shown in the diagram opposite.
Step 6:
C.F(S) = nE.V(S in isolated state) − nE.V(S linked to the building) = 6 − 5 = +1
C.F(O1) = nE.V(O1 in isolated state) – nE.V(O1 bound to the structure) = 6 − 6 = 0 no formal charge on O1
C.F(O2) = nE.V(O2 in isolated state) – nE.V(O2 bound to the structure) = 6 − 7 = −1
C.F(SO2) = C.F(S) + C.F(O1) + C.F(O2) = 1 + 0 − 1 = 0
Step 7:
Build the structure using the systematic method:
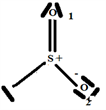
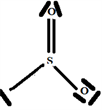
2.6. The Species H2SO3
Step 1:
nE.V(H2SO3) = nE.V(S) + 3nE.V(O) + 2nE.V(H) − 0
Z(S) = 16 hence nE.V(S) = 6; Z(H) = 1 hence nE.V(H) = 1
and Z(O) = 8 hence nE.V(O) = 6
nE.V(H2SO3) = 6 + 3 × 6 + 2 × 1 − 0 = 26
Step 2:
nD(H2SO3) = 1/2 × nE.V(H2SO3) = 26/2 = 13 binding and non-binding doublets.
Step 3:
nD.A.L(H2SO3) = nD.N.L(S) + 3nD.N.L(O) + 2nD.N.L(H) − 0
Z(S) = 16 hence nD.N.L(S) = 2; Z(H) = 1 hence nD.N.L(H) = 0
and Z(O) = 8 hence nD.N.L(O) = 2
nD.A.L(H2SO3) = 2 + 3 × 2 + 2 × 0 − 0 = 8 free doublets.
Step 4:
nD.L(H2SO3) = nD(H2SO3) – nD.A.L(H2SO3)
so nD.L(H2SO3) = 13 − 8 = 5 Bonding Doublets.
Step 5:
The V.S.E.P.R formulation is expressed as follows: AXnEn'’:
S: central atom.
O: three oxygen atoms covalently bonded to the S atom and n = 3 is the number of covalent bonds that oxygen can form with sulfur.
E: two free doublets on the valence layer of atom S.
So the Gillespie type of molecule is H2SO3E2 and the energetically most favorable trigonal bipyramid base geometry in T, as shown in the diagram opposite.
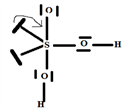
Step 6:
C.F(S) = nE.V(S in isolated state) – nE.V(S linked to the building) = 6 − 6 = 0
C.F(O1) = nE.V (O1 in isolated state) – nE.V(O1 bound to the structure) = 6 − 6 = 0 no formal charge on O1
C.F(O2) = nE.V(O2 in isolated state) – nE.V(O2 bound to the structure) = 6 − 6 = 0 no formal charge on O2
C.F(O3) = nE.V(O3 in isolated state) – nE.V(O3 bound to the structure) = 6 − 6 = 0 no formal charge on O3
C.F(H) = nE.V(H in isolated state) – nE.V(H linked to the building) = 1 − 1 = 0 no formal charge on twice H
C.F(H2SO3) = C.F(S) + C.F(O1) + C.F(O2) + C.F(O3) + 2 C.F(H) = 0 + 3 × 0 + 2 × 0 = 0
Step 7:
Build the structure using the systematic method:
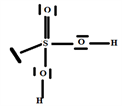
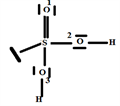
2.7. The Species
Step 1:
nE.V(
) = 2nE.V(Cr) + 7nE.V(O) – (−2)
Z(Cr) = 24 where Cr’s valence layer is: [Ar] 3d5 4s1 → nE.V(Cr) = 6;
Z(O) = 8 from which the valence layer of O is: [He] 2s2 2p4 nE.V(O) = 6;
nE.V(
) = 2 × 6 + 7 × 6 − (−2) = 56
Step 2:
nD(
) = 1/2 × nE.V(
) = 56/2 = 28 binding and non-binding doublets.
Step 3:
nD.A.L(
) = 2nD.N.L(Cr) + 7nD.N.L(O) – (−2)
Z(Cr) = 24 hence nD.N.L(Cr) = 1 and Z(O) = 8 hence nD.N.L(O) = 2
nD A L(
) = 2 × 1 + 7 × 2 – (−2) = 18 free doublets.
Step 4:
nD.L(
) = nD(
) – nD.A.L(
)
so nD.L(
) = 28 − 18 = 10 Bonding Doublets.
Step 5:
The V.S.E.P.R formulation is expressed as follows: AXnEn':
Cr: central atom.
O: four oxygen atoms covalently bonded to the Cr atom and n = 4 is the number of covalent bonds that oxygen can form with chromium.
E: one free doublet on the valence layer of the Cr atom.
The Gillespie type of molecule is therefore CrO4E and the basic geometry trigonal bipyramid, as shown in the diagram opposite.
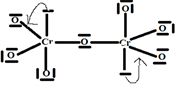
Step 6:
C.F(Cr) = nE.V(Cr in isolated state) − nE.V(Cr bound to the structure) = 6 − 5 = +1
C.F(O1) = nE.V(O1 in isolated state) – nE.V(O1 bound to the structure) = 6 − 6 = 0 no formal charge on O1
C.F(O2) = nE.V(O2 in isolated state) − nE.V(O2 bound to the structure) = 6 − 6 = 0 no formal charge on O2
C.F(O3) = nE.V(O3 in isolated state) – nE.V(O3 bound to the structure) = 6 − 7 = −1
C.F(O4) = nE.V(O4 in isolated state) – nE.V(O4 bound to the structure) = 6 − 7 = −1
C.F(
) = 2C.F (Cr) + C.F(O1) + 2C.F(O2) + 2C.F(O3) + 2C.F(O4) = 2 × 1 + 1 × 0 + 2 × 0 +2 × (−1) + 2 × (−1)
C.F(
) = −2
Step 7:
Build the structure using the systematic method:
![]()
![]()
2.8. The Species Fe3O4
Step 1:
nE V(Fe3O4) = 3nE.V(Fe) + 4nE.V(O) – 0
Z(Fe) = 26 from which the valence layer of Fe is: [Ar] 3d6 4s2 → nE.V(Cr) = 8;
Z(O) = 8 from which the valence layer of O is: [He] 2s2 2p4 → nE.V(O) = 6;
nE.V(Fe3O4) = 3 × 8 + 4 × 6 − 0 = 48
Step 2:
nD(Fe3O4) = 1/2 ×nE.V(Fe3O4) = 48/2 = 24 binding and non-binding doublets.
Step 3:
nD.A.L(Fe3O4) = 3nD.N.L(Fe) + 4nD.N.L(O) – 0
Z(Fe) = 26 from where nD.N.L(Fe) = 2 and Z(O) = 8 from where nD.N.L(O) = 2
nD.A.L(Fe3O4) = 3 ×2 + 4 × 2 – 0 = 14 free doublets.
Step 4:
nD.L(Fe3O4) = nD(Fe3O4) – nD.A.L(Fe3O4)
Therefore nD.L(Fe3O4) = 24 − 14 = 10 Bonding doublets.
Step 5:
The V.S.E.P.R formulation is expressed as follows: AXnEn':
Fe1: central atom
Two oxygen atoms and one Fe2 atom covalently bonded to the Fe1 atom and n = 3 is their number.
E: two free doublets on the valence layer of the Fe1 atom.
The Gillespie type of molecule is therefore Fe1O2Fe2E2 and the basic geometry trigonal bipyramid.
Fe2: central atom
Two iron atoms and one oxygen atom covalently bonded to the central Fe2 atom and n = 3 their number.
E: two free doublets on the valence shell of the central Fe atom.
The Gillespie type of molecule is therefore Fe2Fe1Fe3OE2 and the basic geometry trigonal bipyramid, as shown in the diagram opposite.
![]()
Step 6:
C.F(Fe1) = nE.V(Fe1 in isolated state) − nE.V(Fe1 bound to the structure) = 8 − 8 = 0
C.F(Fe2) = nE.V(Fe2 in isolated state) − nE.V(Fe2 bound to the structure) = 8 − 8 = 0
C.F(Fe3) = nE.V(Fe3 in isolated state) − nE.V(Fe3 bound to the structure) = 8 − 8 = 0
C.F(O) = nE.V(O in isolated state) − nE.V(O bound to the structure) = 6 − 6 = 0 no formal charge on the three O.
C.F(Fe3O4) = 3C.F(Fe) + 4C.F(O) = 3 × 0 + 4 × 0
C.F(Fe3O4) = 0
Step 7:
Build the structure using the systematic method: same layout as described for geometry.
2.9. The Species Pb3O4
Step 1:
nE.V(Pb3O4) = 3 nE.V(Pb) + 4nE.V(O) – 0
Z(Pb) = 82 from which the valence layer of Pb is [Xe] 4f14 5d10 6s26p2 → nE.V(Pb) = 4;
Z(O) = 8 from which the valence layer of O is: [He] 2s2 2p4 → nE.V(O) = 6;
nE.V(Pb3O4) = 3 × 4 + 4 × 6 − 0 = 36
Step 2:
nD(Pb3O4) = 1/2 × nE.V(Pb3O4) = 36/2 = 18 binding and non-binding doublets.
Step 3:
nD.A.L(Pb3O4) = 3nD.N.L(Pb) + 4nD.N.L(O) – 0
Z(Pb) = 82 from where nD.N.L(Pb) = 1 and Z(O) = 8 from where nD.N.L(O) = 2
nD.A.L(Pb3O4) = 3 × 1 + 4 × 2 – 0 = 11 free doublets.
Step 4:
nD.L(Pb3O4) = nD(Pb3O4) – nD.A.L(Pb3O4)
so nD.L(Pb3O4) = 18 − 11 = 7 Bonding Doublets.
Step 5:
The V.S.E.P.R formulation is expressed as follows: AXnEn':
Pb1: central atom
two oxygen atoms and one Pb2 atom covalently bonded to the
central Pb1 atom and n = 3 in number.
E: zero free doublet on the valence layer of the central atom Pb1.
So the Gillespie type of molecule is Pb1O2Pb2 and the basic geometry triangular planar.
Pb2: central atom
two lead atoms and one oxygen atom covalently bonded to the central Pb2 atom and n = 3 their number.
E: zero free doublets on the valence layer of the central atom Pb2.
So the Gillespie type of molecule is Pb2Pb1Pb3O and the basic geometry triangular planar, as shown in the diagram opposite.
![]()
Step 6:
C.F(Pb1) = nE.V(Pb1 in isolated state) − nE.V(Pb1 linked to the building) = 4 − 4 = 0
C.F(Pb2) = nE.V(Pb2 in isolated state) − nE.V(Pb2 linked to the building) = 4 − 4 = 0
C.F(Pb3) = nE.V(Pb3 in isolated state) − nE.V(Pb3 linked to the building) = 4 − 4 = 0
C.F(O) = nE.V(O in isolated state) − nE.V(O bound to the structure) = 6 − 6 = 0 no formal charge on the three O
C.F(Pb3O4) = 3C.F(Pb) + 4 C.F(O) = 3 × 0 + 4 × 0
C.F(Pb3O4) = 0
Step 7:
Build the structure using the systematic method:
![]()
2.10. The Species H2O2
Step 1:
nE.V(H2O2) = 2nE.V(O) + 2nE.V(H) − 0
Z(O) = 8 from where nE.V(O) = 6 and Z(H) = 1 from where nE.V(H) = 1
nE.V(H2O2) = 2 × 6 + 2 × 1 − 0 = 14
Step 2:
nD(H2O2) = 1/2 ×nE.V(H2O2) = 14/2 = 7 binding and non-binding doublets.
Step 3:
nD.A.L(H2O2) = 2nD.N.L(O) + 2nD.N.L(H) − 0
Z(O) = 8 from where nD.N.L(O) = 2 and Z(H) = 1 from where nD.N.L(H) = 0
nD.A.L(H2O2) = 2 × 2 + 2 × 0 – 0 = 4 free doublets.
Step 4:
nD.L(H2O2) = nD(H2O2) – nD.A.L(H2O2)
Therefore nD.L(H2O2) = 7 − 4 = 3 Bonding Doublets.
Step 5:
The V.S.E.P.R formulation is expressed as follows: AXnEn':
O: central atom.
An oxygen atom and a hydrogen atom covalently bonded to the O atom and n = 2 is the number of covalent bonds that oxygen 1 can form with oxygen 2 and hydrogen.
E: free doublets and 2 their number on the valence layer of the O1 atom.
So the Gillespie type of molecule is O1H1E2 and the basic geometry is tetrahedral angled, as shown in the diagram opposite.
![]()
Step 6:
C.F(O) = nE.V(O in isolated state) – nE.V(O bound to the structure) = 6 − 6 = 0 no charge for both oxygens
C.F(H) = nE.V(H in the isolated state) – nE.V(H bound to the structure) = 1 − 1 = 0 no charge for the two hydrogens
C.F(H2O2) = 2C.F(O) + 2C.F(H) = 2 × 0 + 2 × 0 = 0
Step 7:
Build the structure using the systematic method:
![]()
3. Oxidation Number of Atoms in Buildings Using the Systematic Method
We determine the degree of oxidation of the various atoms in the structure using the systematic method for some of the following molecules:
;
;
; PCl5; SO2; H2SO3;
; Fe3O4; Pb3O4; H2O2.
3.1. The Species
Step 8:
We have χ(N) > χ(H), so the nitrogen atom will attract towards itself the electrons involved with hydrogen.
d.o(N) = nE.V(N atom in isolated state) – nE.V(N atom bound to the structure) = 5 − 8 = −3
d.o(H) = nE.V(H atom in isolated state) – nE.V(H atom bound to the structure) = 1 − 0 = 1
C.F(
) = d.o(N) + 4d.o(H) = −3 + 4 × 1 = +1
3.2. The Species
Step 8:
We have χ(N) > χ(H), so the nitrogen atom will attract towards itself the electrons involved with hydrogen.
d.o (N) = nE.V (N atom in isolated state) – nE.V (N atom bound to the structure) = 5 − 8 = −3
d.o (H) = nE.V (H atom in isolated state) – nE.V (H atom bound to the structure) = 1 − 0 = 1
C.F (
) = d.o (N) + d.o (H1) + d.o (H2) = −3 + 2 × 1 = −1
3.3. The Species
We have χ(O) > χ(N), so the oxygen atom will attract to itself the electrons involved with nitrogen.
d.o(N) = nE.V(N atom in isolated state) – nE.V(N atom bound to the structure) = 5 − 2 = 3
d.o(O1) = nE.V(O atom1 in isolated state) – nE.V(O atom1 bound to the edifice) = 6 − 8 = −2
d.o(O2) = nE.V(O atom2 in isolated state) – nE.V(O atom2 bound to the edifice) = 6 - 8 = - 2
C.F(
) = d.o(N) + d.o(O1) + d.o(O2) = 3 + 2 × (−2) = −1
3.4. The Species PCl5
We have χ(Cl) > χ(P), so the chlorine atom will attract to itself the electrons involved with the phosphorus.
d.o(Cl) = nE.V(Cl atom in isolated state) – nE.V(Cl atom bound to the structure) = 7 − 8 = −1
d.o(P) = nE.V(P atom in isolated state) − nE.V(P atom bound to the structure) = 5 − 0 = +5
C.F(PCl5) = d.o(S) + 5d.o(Cl) = 5 + 5 × (−1) = 0
3.5. The Species SO2
We have χ(O) > χ(S), so the oxygen atom will attract to itself the electrons involved with sulfur.
d.o(S) = nE.V(S atom in isolated state) – nE.V(S atom bound to the structure) = 6 − 2 = +4
d.o(O1) = nE.V(O atom1 in isolated state) – nE.V(O atom1 bound to the edifice) = 6 − 8 = −2
d.o(O2) = nE.V(O atom2 in isolated state) – nE.V(O atom2 bound to the edifice) = 6 − 8 = −2
C.F(SO2) = d.o(S) + d.o(O1) + d.o(O2) = 4 + 2 × (−2) = 0
3.6. The Species H2SO3
We have χ(O) > χ(S) and χ(O) > χ(S), so the oxygen atom will attract the electrons involved with sulfur and hydrogen.
d.o(S) = nE.V(S atom in isolated state) – nE.V(S atom bound to the structure) = 6 − 2 = +4
d.o(O1) = nE.V(O atom1 in isolated state) – nE.V(O atom1 bound to the edifice) = 6 − 8 = −2
d.o(O2) = nE.V(O atom2 in isolated state) – nE.V(O atom2 bound to the edifice) = 6 − 8 = −2
d.o(O3) = nE.V (O atom3 in isolated state) – nE.V(O atom3 bound to the edifice) = 6 − 8 = −2
d.o(H) = nE.V (H atom in isolated state) – nE.V(H atom bound to the structure) = 1 − 0 = +1
C.F(H2SO3) = d.o(S) + d.o(O1) + d.o(O2) + d.o(O3) + 2d.o(H) = 4 + 3 × (−2) + 2 × 1 = 0
3.7. The Species
We have χ(O) > χ(Cr), so the oxygen atom will draw the electrons involved with chromium towards itself.
d.o(Cr) = nE.V(Cr atom in the isolated state) – nE.V(Cr atom bound to the structure) = 6 − 0 = + 6
d.o(O1) = nE.V(O atom1 in isolated state) - nE.V(O atom1 bound to the edifice) = 6 − 8 = −2
d.o(O2) = nE.V(O atom2 in isolated state) – nE.V(O atom2 bound to the edifice) = 6 − 8 = −2
d.o(O3) = nE.V(O atom3 in isolated state) – nE.V(O atom3 bound to the edifice) = 6 − 8 = −2
d.o(O4) = nE.V(O atom4 in isolated state) – nE.V(O atom4 bound to the edifice) = 6 − 8 = −2
C.F(
) = 2d.o(Cr) + d.o(O1) + 2d.o(O2) + 2d.o(O3) + 2d.o(O4) = 2 × 6 + 1 × (−2) + 2 × (−2) + 2 × (−2)
C.F(
) = −2
3.8. The Species Fe3O4
We have χ(O) > χ(Fe), so the oxygen atom will attract towards itself the electrons involved with the iron.
d.o(Fe1) = nE.V(Fe atom1 in isolated state) – nE.V(Fe atom1 bound to the structure) = 8 − 5 = +3
d.o(Fe2) = nE.V(Fe atom2 in isolated state) – nE.V(Fe atom2 bound to the structure) = 8 − 6 = +2
d.o(Fe3) = nE.V(Fe atom3 in isolated state) – nE.V(Fe atom3 bound to the structure) = 8 − 5 = +3
d.o(O) = nE.V(O atom in isolated state) – nE.V(O atom bound to the structure) = 6 − 8 = −2
C.F(Fe3O4) = d.o(Fe1) + d.o(Fe2) + d.o(Fe3) + 4d.o(O) = 3 + 2 + 3 + 4 × (−2) C.F(Fe3O4) = 0
3.9. The Species Pb3O4
We have χ(O) > χ(Pb), so the oxygen atom will attract towards itself the electrons involved with lead.
d.o(Pb1) = nE.V(Pb atom1 in isolated state) – nE.V(Pb atom1 bound to the structure) = 4 − 1 = +3
d.o(Pb2) = nE.V(Pb atom2 in isolated state) – nE.V(Pb atom2 bound to the structure) = 4 − 2 = +2
d.o(Pb3) = nE.V(Pb atom3 in isolated state) – nE.V(Pb atom3 bound to the structure) = 4 − 1 = +3
d.o(O) = nE.V(O atom in isolated state) – nE.V(O atom bound to the structure) = 6 − 8 = −2
C.F(Pb3O4) = d.o(Pb1) + d.o(Pb2) + d.o(Pb3) + 4d.o(O) = 3 + 2 + 3 + 4 × (−2) C.F(Pb3O4) = 0
3.10. The Species H2O2
We have χ(O) > χ(H), so the oxygen atom will attract towards itself the electrons brought into play with hydrogen.
d.o(O1) = d.o(O2) = nE.V(O atom in isolated state) - nE.V(O atom bound to the structure) = 6 − 7 = −1
d.o(H1) = d.o(H2) = nE.V(H atom in isolated state) – nE.V(H atom bound to the structure) = 1 − 0 = +1
C.F(H2O2) = 2d.o(O) + 2d.o(H) = 2 × (−1) + 2 × 1 = 0
The importance of this systematic method for the Lewis representation of molecules lies in determining the oxidation state of each atom in the structure [7] - [12] .
4. Conclusion
The systematic method for constructing Lewis representations of molecules is an excellent method for simultaneously determining the Gillespie geometry, formal charge and oxidation state of molecules, and is better than the other methods Lewis representations of atoms as well as the linear combination method of atomic orbitals [1] - [6] .