1. Introduction
A healthy immune system can defeat invading disease-causing pathogens as well as cancer cells. However, when the number of pathogens is overwhelming or there is any defect in the immune system such as defects in antibody production, complement and neutrophils, infections can occur [1] . Individuals who are immunocompromised have reduced ability to fight infections and other diseases [2] . This inability to fight infection may be caused by diseases or conditions such as HIV/AIDS, cancer, diabetes mellitus and so on [3] .
Diabetic patients’ immune response is disrupted as a result of the prevailing hyperglycemic condition. In addition to the risk of natural barrier damage due to neuropathy, diabetes especially type 2 can also affect cellular immunity [4] . Diabetes mellitus results from the inability of β-cells in the pancreas to produce sufficient insulin or the insulin produced cannot be properly utilized [5] . According to the American Diabetes Association (ADA), infections are an important issue for individuals with diabetes due to the failure of the immune system to fight off invading pathogens [6] .
People living with diabetes are more susceptible to infections than people without diabetes [7] . One of the commonest infections in these immunocompromised diabetic patients is UTI [8] . This results from decreased cellular responses and poor pathogen clearance that are very common in these patients [9] . Urinary tract infection is a major cause of death in these groups of immunocompromised patients [9] .
Studies have reported E. coli to be the commonest bacterial uropathogens of UTIs [10] . This is due to the presence of a number of virulence [11] . and because E. coli is a common flora in the gastrointestinal tract, rectal and vagina area from where it could ascend to the urinary tract.
Bacteria that are resistant to multiple antimicrobial agents are called multi-drug resistant (MDR), those with extensive drug resistant (XDR) or totally drug resistant (TDR) are sometimes called “superbugs” [12] . The study was undertaken to determine the prevalence of drug resistance uropathogenic E. coli from diabetic patients with hyperglycemic condition attending three selected health facilities in Benue State.
2. Materials and Methods
2.1. Study Design, Area and Population
This study used a cross-sectional survey design carried out from November, 2021 to April, 2022. It includes patients seeking medical services at Federal Medical Centers, Makurdi, General Hospital at Vandeikya and Otukpo, Benue State. It includes both in-patients and out-patients showing symptoms of urinary tract infections. Patients selected for the study and control group were diabetic patients with blood glucose levels ≥ 200 mg/100ml and between 126 mg/100ml and 200 mg/100ml respectively, who were not on any antibiotic therapy for the past two weeks before the study period. Patients with extreme age below 18 and above 60 years were excluded.
A total of 296 diabetes mellitus patients with blood glucose levels ≥ 11.1 mmol/L (200 mg/dl) were the test and other 296 diabetes patients with blood glucose levels between 7.0 - 11.1 mmol/L (126 mg/dl and 200 mg/dl) were the control. Sample size was determined using the simple population proportion formula described by Charan and Biswas [13] .
where n = the study sample size,
Z = Z score for 95% confidence interval (degree of confidence) = 1.96
P = prevalence of UTI in the study area (26.03%) [14] .
d = margin of error (of setting a significant level of 0.05) = 5% (0.05)
The participants for both studies were selected using systematic random sampling [15] .
2.2. Ethical Clearance
Ethical approval was obtained from the Health Research Ethical Committee at Federal Medical Centre (FMH/FMC/MED/108/VL.1) and Benue State Hospitals Management Board, Makurdi (HMB/OFF/215/VOL.II/455). Permission was also obtained from the Principal Medical Officer of the respective health care facilities. Informed written consent was obtained from all the study participants. Questionnaire was also given to each patient to obtain demographic and clinical information.
2.3. Bacterial Isolation
Midstream urine samples from patients were cultured on Cysteine Lactose Electrolyte Deficient (CLED) (Oxoid, CM 0398), MacConkey and Blood Agar (Oxoid) to determine the bacterial uropathogens present in each urine sample. Each urine sample was aseptically inoculated onto the culture media and incubated aerobically at 37˚C for 24 hours.
The media which show no bacteria growth after 24-hours incubation were further incubated up to 48 hours before reporting no bacterial growth. The numbers of isolated bacterial colonies were. Only urine samples that gave single pure with colony ≥ 105 CFU/ml were taken as significant growth [16] [17] .
2.4. Identification of Bacterial Uropathogens
E. coli isolates were identified following standard bacteriological procedures as described by Cheesbrough [16] , Kolawale et al. [18] and through molecular approaches as reported by Adeoti [19] .
2.5. Antibiotic Susceptibility Testing (AST)
The isolates were subjected to in-vitro antibiotic susceptibility testing using the modified Kirby-Bauer disc diffusion method according to the guidelines recommended by the CLSI [20] . Antibiotics used in this study were Mast discs product. They included ampicillin (AMP) 10 μg, cefoxitin (FOX) 30 μg, cefotaxime, (CTX) 30 µg, ceftriaxone (CRO) 30 μg, imipenem (IMI) 10 µg, aztreonam (ATM) 30 μg, augmentin (AUG) (amoxicillin/clavulinic acid 20/10μg), genticn (GM) 10 μg, streptomycin (S) 10 µg, chloramphenicol (C) 30 μg, cotrimoxazole (trimethoprim-sulfamethoxazole 1.25/23.75) (COT) 25 μg, nalidixic acid (NA) 30 µg, ciprofloxacin (CIP) 5 µg, ofloxacin (OFX) 5 µg, levofloxacin (LEV) 5 µg, nitrofurantoin (NI) 300 µg and tetracycline (T) 30 μg.
Antibiotic susceptibility testing was done on a sterile Muller Hinton Agar (Oxoid, CM00339) plate using an equivalent of 0.5% McFarland standard (108 CFU/mL), incubated and interpreted as recommended by the CLSI [20] .
2.6. Identification of Drug Resistant Isolates
All drug resistant uropathogenic E. coli isolates were identified as multi-drug resistant isolates (MDR), extensive drug resistant isolates (XDR) and pan-drug resistant isolates following the combined guidelines of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC). The E. coli were identified as MDR when resistant to 1 agent in 3 antibiotic classes. They were extensively drug resistant organisms (XDR) when non-susceptibility to at least one agent in all but two or fewer antibacterial categories. Pan-drug resistant organisms (PDR) were defined as non-susceptibility to all agents in all antibiotic classes [21] .
2.7. Statistical Analysis
Data generated were analyzed using SPSS software version 20.0 for Windows. Chi-square test was used to assess and compare relationships between variables. In all the comparisons, p-value less than 0.05 were taken as statistically significant levels. Sample size was determined using the formula [12]
3. Results
3.1. Prevalence of Urinary Tract Infections
The overall prevalence of UTIs in this study was 20.9%. Female diabetic patients had the highest occurrence of UTI (54.8%) (Table 1). Age group 46 and 50 years had the highest cases 21 (33.9%), followed by age group 51 and 55, 16 (25.8%), age group 41 and 45 years 9 (14.5%), age groups 36 - 40 and 56 - 60 years had 8 (12.9%) each, and all participants in the age groups 18 - 25, 26 - 30 and 31 - 35 had no positive UTI case. The female participants in the age group 46 and 50 years had the highest occurrence of UTIs (12), followed by the males in the age groups 46 - 50 and 51 - 55 years each having 9 while age group 41 and 45 of the male participants had the lowest with only a patient (Figure 1).
![]()
Table 1. Socio-demographic characteristics and prevalence of UTI in the studied DM patients.
Key: χ2: Chi-square %: percent, UTI: Urinary tract infection and DM: Diabetic mellitus.
The married participants had the highest occurrence of UTI 58 (93.5%), followed by the widowed 3 (0.5%) and single. Participants who are more educated gave the highest occurrence of UTI 29 (26.8%), while the illiterates showed the least occurrence of UTI 7 (11.3%). Urban dwellers patients had UTI of 37 (59.7%) while 25 (40.3%) lived in rural areas. Farmers and house wives had the highest occurrence of UTIs 17 (27.4%), followed by civil servants 15 (24.2%), business owners 12 (19.4%) and students 1 (1.6%). There was significant association between occurrence of UTIs and occupation (p = 0.046) of the enrolled diabetic patients (Table 1).
3.2. Bacterial Profile of UTI in Immunocompromised Diabetic Patients
Out of the total number of urine samples analyzed, 62 (20.9%) and 25 (8.4%) of them were positive for bacterial culture for the study population and control subject respectively (Table 2). From the study, 14, 20 and 28 E. coli isolates were identified from Vandeikya, Otukpo and Makurdi location respectively.
3.3. Antibiotic Susceptibility Pattern of Uropathogenic E. coli Isolates
Antibiotic susceptibility test was performed for all the E. coli isolates using 17 different antibiotics. From Vandeikya location, the isolates showed high sensitivity to cefotaxime 12 (85.7%), followed by imipenem 10 (71.4%), chloramphenicol 9 (64.3%), nitrofurantoin 9 (64.3%), ceftriaxone 9 (64.3%), moderate susceptibility to genticin, streptomycin, tetracycline and ciprofloxacin. There was no sensitivity to ampicillin, aztreonam and nalidixic acid.
Escherichia coli isolates from the health facilities at Otukpo location demonstrated
![]()
Figure 1. Age/sex specific prevalence of UTIs from diabetic patients in the study area.
![]()
Table 2. Bacterial profiles in the diabetes mellitus patients.
high susceptibility to streptomycin 12 (60%), cefotaxime 11 (55%) and imipenem 10 (50%). High resistance was exhibited to ampicillin 16 (80%), aztreonam 16 (80%), levofloxacin 16 (80%), nalidixic acid 14 (70%), ofloxacin 14 (70%), ciprofloxacin 13 (65%), ceftriaxone 12 (60%) and tetracycline 11 (55%). Escherichia coli from Makurdi were most susceptible to streptomycin (75%) followed by cefotaxime (64.3%), and no susceptibility to ampicillin and aztreonam. Similarly, the rate of resistance ranged from ampicillin 89.3%, aztreonam 78.6%, nalidixic acid 71.4%, cefoxitin 57.1%, ofloxacin 53.6%, levofloxacin 53.6%, and tetracycline 50% while other antibiotics yielded less than 50% resistance pattern (Table 3).
3.4. Percentage of Isolated E. coli Showing Resistance to the Different Antibiotics Used in This Study
The percentile distribution (Figure 2) showed the prevalence of resistance of
![]()
Table 3. Antibiotic susceptibility pattern of bacterial uropathogens from diabetes mellitus patients in the study area.
Key: AMP: Ampicillin, FOX: Cefoxitin, CTX: Cefotaxime, CRO: Ceftriaxone, IMI: Imipenem, ATM: Aztreonam, AUG: Augmentin, GM: Genticin, S: Streptomycin, T: Tetracycline, C: Chloramphenicol, ATH: Azithromycin, E: Erythromycin, COT: Cotrimoxazole, NA: Nalidixic acid, CIP: Ciprofloxacin, OFX: Ofloxacin, LEV: Levofloxacin, NI: Nitrofurantoin, S: Sensitive, I: Intermediate, R: Resistant.
E. coli isolates to the different classes of antibiotics used. The isolated E. coli gave the highest percentage resistance to ampicillin (89.3%) in Makurdi, followed by nalidixic acid (85.7%) in Vandeikya, ampicillin, aztreonam and levofloxacin from Otukpo 80%. Escherichia coli demonstrated the lowest percentage resistance to cefotaxime (14.3%) in Vandeikya, followed by imipenem (21.4%) in Vandeikya and cefotaxime (21.4%) in FMC, Makurdi (Figure 2).
3.5. Occurrence of MDR, XDR and PDR Isolates among Uropathogenic E. coli Isolates
Out of the thirty-eight (38) drug resistant E. coli isolates detected from study group (diabetic patients with hyperglycemic condition), 25 were MDR strains, 8 XDR strains and 5 PDR strains. The 25 MDR isolates were distributed as follows: 8 (21.0%) isolates from Vandeikya, 8 (21.0%) isolates from Otukpo and 9 (23.7%) from Makurdi. One (2.6%) XDR isolates were from Vandeikya, 3 (7.9%) from Otukpo and 4 (10.6%) from Makurdi while 2 (5.3%) and 3 (7.9%) were positive PDR strains from Otukpo and Makurdi respectively (Figure 3).
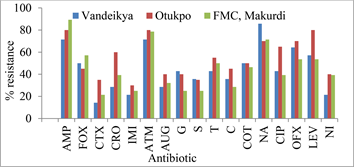
Key: AMP: Ampicillin, FOX: Cefoxitin, CTX: Cefotaxime, CRO: Ceftriaxone, IMI: Imipenem, ATM: Aztreonam, AUG: Augmentin, GM: Genticin, S: Streptomycin, T: Tetracycline, C: Chloramphenicol, ATH: Azithromycin, E: Erythromycin, COT: Cotrimoxazole, NA: Nalidixic acid, CIP: Ciprofloxacin, OFX: Ofloxacin, LEV: Levofloxacin and NI: Nitrofurantoin.
Figure 2. Percentage of uropathogenic E. coli showing resistance to the different antibiotics used in the three different locations.
![]() Key: VD: Vandeikya, OP: Otukpo, FMC: Federal Medical Centre, Makurdi, Ec: E. coli, MDR: multi-drug resistance, XDR: Extensive drug resistance, PDR: Pan-drug resistance.
Key: VD: Vandeikya, OP: Otukpo, FMC: Federal Medical Centre, Makurdi, Ec: E. coli, MDR: multi-drug resistance, XDR: Extensive drug resistance, PDR: Pan-drug resistance.
Figure 3. Occurrence of MDR, XDR and PDR isolates in the isolated uropathogenic E. coli from diabetic patients in the study area.
4. Discussion
The present study was undertaken to ascertain the prevalence of UTI and associated antibiotic resistance mechanisms in these patients. The goal is to determine the most effective approach towards management of UTI to reduce the burden of this infection in this group of immunocompromised patients. The study showed the prevalence of UTI among immunocompromised diabetic patients (blood glucose levels ≥ 11.1 mmol/L) to be higher (20.9%) than the diabetic patients with blood glucose levels between 7.0 mmol/L and 11.1 mmol/L (8.4%).
The prevalence of UTI recorded for diabetes mellitus study population is comparable to the reported prevalence in other studies such as 22.6% recorded in a study in Addis Ababa [22] . and 19.5% among diabetic in Khartoum, Sudan [23] . It was consistent with studies of Forson et al. [24] who reported 9.2%, 10.1% recorded at Derbe Tabor General Hospital, Northwest Ethiopia [25] and 10.5% in Southern Ethiopia [26] . In contrast, much higher prevalence of 35.3% was reported in Iraq [27] 40.2% in Malaysia [28] and 54.7% in Nepal [29] .
The prevalence of UTI based on age group showed that age group 46 and 50 had the highest prevalence (33.9%) while age groups 18 and 25, 26 and 30, 31 and 35 had no recorded positive UTI cases. No significant association was observed in relation to age group of the participants. Study by Al-Rubeaan et al. [30] carried out in Saudi reported similar findings while another study in Sweden by Hamdan et al. [23] reported significant association between age groups and positive UTI cases among diabetic patients. Some studies have reported that as age increases, UTI cases in diabetic patients also increased probably due to decreasing state of immunity of the patients as a result of the hyperglycemic condition [31] .
In this study, UTI occurs only in the age groups 36 - 40, 41 - 45, 46 - 50, 51 - 55 and 56 - 60 with age groups 46 - 50 and 51 - 55 highest. This observation was similar to the studies of Njunda et al. [32] in Cameroon who also recorded high prevalence among age group 41 - 60 years. This was attributed to increased sexual activity in this age group [33] . Contrary to this, Walelgn et al. [34] in South Wollo, Northeast Ethiopia, Chita et al. [35] in the UK and Forson et al. [24] in Ghana obtained higher occurrence of UTIs among diabetic patients in the age group 61 - 70 years.
Farmers and house wives each had prevalence of 27.4% UTI being the highest. There was significant association between occurrence of urinary tract infection and occupation (p = 0.046) of the diabetic patients.
All the E. coli isolates in this study showed resistance to at least one antibiotic and none showed complete susceptibility to all the antibiotics tested. Escherichia coli from the location at Vandeikya were sensitive to β-lactams (cefotaxime and imipenem), chloramphenicol and nitrofurantoin. Slightly higher sensitivity has been reported in a study among diabetic patients in Ethiopia to nitrofurantoin 95%, cefotaxime 85% and ceftriaxone 85%. [22] . Another study in Ethiopia also reported high susceptibility (100%) to nitrofurantoin, genticin and meropenem [25] . This study also reported high resistance to ampicillin 71.4%, ofloxacin 64.3%, cefoxitin 50% and cotrimoxazole 50%. Worku et al. [25] reported similar high resistance pattern to ampicillin 100%, cefoxitin 100%, augmentin 92.9% and cotrimoxazole 61.1% in Ethiopia.
The results of antibiotic susceptibility pattern from Otukpo revealed E. coli to be moderately susceptible to streptomycin 60%, cefotaxime 55% and imipenem 50% while at the same time showing moderate to high resistance to cotrimoxazole 50%, tetracycline 55%, ceftriaxone 60%, ciprofloxacin 65%, ofloxacin 70%, nalidixic acid 70%, levofloxacin 80%, aztreonam 80%, ampicillin 80%. This was contrary to a study in India [36] who reported complete sensitivity (100%) to imipenem but comparable resistant pattern has been observed in Ethiopia to nalidixic acid 70% and cotrimoxazole 64.7% [26] .
The isolated E. coli from Makurdi exhibited moderate to slightly high susceptibility pattern to nitrofurantoin, augmentin, genticin, imipenem and streptomycin which partly agreed with a study in Ethiopia where high sensitivity was demonstrated to nitrofurantoin 95.5% and genticin 73.3% [37] . Another study in Ethiopia also reported 100% sensitivity to imipenem and nitrofurantoin [25] . Escherichia coli also showed moderate to high resistance to tetracycline, levofloxacin, ofloxacin, cefoxitin, nalidixic acid, aztreonam and ampicillin in this study. A similar high resistance pattern was reported in a study conducted among diabetic patients in Jos, Nigeria where resistance to ofloxacin was 70% and ampicillin 90% [38] .
The antibiotic susceptibility test results showed streptomycin and cefotaxime as the most effective antibacterial against E. coli isolates, hence could be used as therapeutic agents in the empirical treatment of UTIs in diabetic patients in the study area. Also, most resistance were expressed to antibacterial agents like ampicillin, cotrimoxazole, tetracycline and the quinolones which might be due to their ready availability, being agents commonly used for self-medication. These antibacterial agents are also commonly used agents for treatment of other bacterial infections.
This study showed high resistance to older quinolone, nalidixic acid than to newer quinolones like ciprofloxacin, ofloxacin and levofloxacin. Findings by Woldemariam et al. [22] reported similar observations in their study. Quinolone antibiotics like ciprofloxacin, ofloxacin and levofloxacin are commonly prescribed antibiotics in this country for the treatment of UTIs in most diabetic patients. This could be one of the major reasons the bacteria isolate from diabetic patients in this study are resistant to these antibiotics.
Relatively low rates of resistance were expressed to the 2 aminoglycosides, genticin and streptomycin used in this study. Studies in Nigeria [39] , Ethiopia [40] [41] have all reported comparable antibiogram pattern to these antibiotics. The study also demonstrated the low resistance to cefotaxime which disagrees with a study in Iran [42] where the E. coli isolates exhibited the highest resistance to cefotaxime. This low resistance exhibited to the third-generation cephalosporin could be because they are not among the commonly used antibiotics for the treatment of UTIs in most communities in the study area. It could also be as a result of their non-availability and the high cost of purchasing them.
This study showed 38 drug resistant Escherichia coli isolates among the study diabetic patients consisting of 25 MDR, 8 XDR and 5 PDR isolates. This implies danger with respect to the global fight to reduce antibiotic resistance by organisms. The findings are comparable to that of Aly and Balkhy [43] who reported similar isolates of E. coli as the most common MDR isolates. It also correlates well with another study conducted in a tertiary care hospital, Central India [44] where E. coli was reported to be most predominant MDR pathogens followed by K. pneumoniae. Studies by Abbas et al. [45] from Pakistan and Effah et al. [46] reported contrary findings of K. pneumoniae isolates as most common XDR strains.
The high number of MDR, XDR and PDR isolates in this study could be a possible reflection of the increasing antibiotic resistance among the uropathogens. The health facilities used for this study are all referral centers that attend to patients who may have been treated with one antibiotic or the other from smaller health facilities scattered all around the different study health centres.
One major limitation of the study is that it was conducted in Benue State, north-central Nigeria instead of all the states of the federation. The selected immunocompromised patients were diabetes mellitus patients with blood glucose levels ≥ 200 mg/dl (11.1 mmol/L). The study was restricted to this group of immunocompromised patients seeking medical attention and those on hospital admission in Federal Medical Centre (FMC), Makurdi, General Hospitals at Otukpo and Vandeikya all within the State. The study focused only on one bacterial pathogen (E. coli) excluding other bacterial uropathogens, and other pathogens such as fungi and protozoan which might have added more value to the research. Other useful clinical and laboratory tests like HbA1c estimations, cytokine profile and possibly white blood cell counts were not included. The study did not provide information about extended spectrum β-lactamase (ESBL) production of the isolated E. coli. Besides, the study did not conduct detailed study on the isolates from the control group just as it was done on those from the study diabetic group.
5. Conclusion
High levels of resistance were expressed to ampicillin, aztreonam, nalidixic acid. Multidrug resistant, XDR and PDR urinary E. coli isolates were also high. However, the isolates were susceptible to streptomycin and cefotaxime thereby making these drugs useful for UTI treatment in immunocompromised diabetic patients.