Effects of Agro-Ecological Practices on the Productivity of Orange-Fleshed Sweet Potato (Ipomoea batatas (L.) Lam) and Soil Fertility in the Sudano-Sahelian Zone of Burkina Faso ()
1. Introduction
Sweet potato is the seventh most important crop worldwide and the fifth most important in developing countries after rice, wheat, maize and cassava [1] . Global sweet potato production in 2017 stood at 112 million tons on a total cultivated area of 9.20 million ha [2] . In Africa, total production is 28 million tons, or 20% of global production [2] . Yields vary widely according to geographical area, ranging from over 21 t/ha in China to 5 t/ha in Africa [2] . It is a major source of income for women, who are heavily involved in post-harvest activities (transformation and marketing). Sweet potatoes are mainly used for human consumption, due to their high nutritional value. It contains 20% - 27% starch and 5% - 8% simple sugars, 0.3% - 8.1% protein, vitamin A, vitamin C, riboflavin, calcium, antioxidants and dietary fiber [3] [4] [5] . Tubers are also a good source of calories (105.3 kcal per 100 g), which the body needs and which give vitality and energy [6] . Varieties of sweet potato can help prevent vitamin A deficiency, particularly in children and pregnant women [7] .
Agronomically, well-drained sandy-loam or sandy-loam soils are favorable for the cultivation of sweet potatoes. It grows best on aerated soils with pH values between 5 and 7.8 [8] . It is relatively resistant to environmental stress [1] . Sweet potato, like other root and tuber crops, responds to good soil fertility, particularly nitrogen and potassium nutrition [6] [9] [10] [11] [12] [13] . On average, it requires 75 kg N/ha; 25 - 50 kg P/ha; and 75 - 100 kg K/ha [12] . For example, one ton of tuberous roots exports 4.70 Kg of nitrogen; 1.30 Kg of phosphorus; and 7.30 Kg of potash [6] . Sweet potatoes can also be grown on poor soils, but require deep, moist, humus-rich soil [14] . Climatically, temperatures of 15˚C or less give little or no growth [8] . A photoperiod of less than 11 hours can stimulate flowering induction, but a photoperiod of more than 13 hours 30 minutes inhibits flowering [15] .
Sweet potato ranks first in terms of production of the root and tuber crop group among vegetable crops in Burkina Faso [2] . However, yields remain low and stagnant at 11.57 t/ha for a potential of 25 t/ha despite sweet potato cultivation occupying a prominent place in Burkina Faso’s market gardening system [16] . One of the causes of this low yield is poor soil organic matter and phosphorus, which are the main constraints to intensifying production [17] . In addition, the use of mineral fertilizer formulas not adapted to vegetable crops has led to unbalanced inputs and, in the long term, to the accumulation of certain heavy metals in the soil [18] . Even in the few farms where mineral fertilizers are used, low nutrient absorption efficiency, significant nutrient losses due to leaching, nutrient imbalances in the soil, and overall reduced soil health and crop yields have been reported [19] [20] [21] [22] , mostly attributed to low soil organic matter levels, many micronutrient deficiencies and high acidity [23] [24] . Indeed, many studies have shown the negative long-term effects of mineral fertilizers on soil fertility, notably through their acidifying effect on the soil [21] [22] [25] . In addition, the widespread use of chemical products over the long term would lead to pathogen resistance to pesticides and deteriorate the quality of arable land. This damage is not confined to the environment (water, soil, biodiversity and climate), but also affects human health [26] . In response to this situation, vegetable production needs to evolve towards more productive and sustainable methods of cropping systems, and agro-ecological practices that promote the integrated soil fertility management approach offer a sustainable solution to declining soil fertility. Thus, alternative fertilization strategies, like organic fertilizers or other types of organic materials, represent environmentally friendly practices that can contribute to sustainable farming systems [27] [28] [29] . To achieve this, organic fertilizers subjected to processes like composting may be valid options for the sustainability of agriculture and the enhancement of the ecosystem services improvement of soil health, and increment of soil organic carbon stocks [30] [31] . However, the effectiveness of organic matter highly depends on the source, nutrient content, stage of mineralization [32] . The present research was conducted to assess the effects of compost combined or not with wood ash on orange-fleshed.
2. Material and Methods
2.1. Experimental Site
The study was carried out in the village of Lantargou located in the rural commune of Diapangou in the eastern region of Burkina Faso, with geographical coordinates 12˚06'57"N and 0˚11'22"W. Climate is Sudano-Sahelian, characterized by two seasons: a dry season lasting six (06) months from November to April, and a rainy season generally from May to October. Over the last 10 years (2013 to 2022), rainfall in the Diapangou commune has varied from 693.2 in 2018 to 1017 in 2022, with an average of 852.48 ± 94.35 mm (Figure 1(a)). The
![]()
Figure 1. Variation in annual rainfall and number of rainy days from 2013 to 2022 (a); Rainfall and number of rainy days in 2022 (b) of experimental site. Data source: data from the Eastern Region weather station.
number of rainy days was 62 in 2017 against 78 in 2020, with an average of 72 ± 5 rainy days (Figure 1(a)). Rainfall in 2022 varied from 24.6 mm in April to 283.1 mm in August, with a monthly average of 145.29 ± 113.55 mm from April to October (Figure 1(b)). In 2012, the number of rainy days was 4 in April and 19 in August, with an average of 11 ± 6 rainy days (Figure 1(b)).
The vegetation cover is made up of shrub savannah-type formations. Parent materials of soils derived from to stable rock formation 3 billion years old composed of granite, schists and gneiss. The soil database highlights three (3) soil classes: sesquioxide soils, less evolved soils and crude mineral soils according to the French classification [33] . According to the World Reference Base for Soil Resources [34] , the following soil groups are found: Leptic or Lithic Leptosols, Epipetric Plinthosols, Endopetroplinthic Lixisols, Haplic or Vertic Cambisols, Fluvisols and Gleysols. These soils are predominantly acidic and poor in the mineral elements N, P, K Ca, with low water retention capacity.
2.2. Test Crop, Fertilizers and Biopesticides
The plant material used was the JEWEL variety of orange-fleshed sweet potato. This variety is long and irregular in shape, with orange skin and pulp. It has a transplant-to-harvest cycle of 90 days and a potential yield of 15 to 25 t/ha. Compost enriched with powdered bone meal and wood ash from the burnt remains of firewood collected from households was used as organic fertilizers. The chemical characteristics of the fertilizers are shown in Table 1.
The biopesticide used was a macerated solution of neem seed powder, garlic, onion, pepper and soap. In fact, one kg of neem seed powder, one kg of pepper, onion and garlic were also ground using a mortar, followed by a ball of grated soap. All these ingredients were mixed in five (05) liters of water and left to macerate for 24 hours. After 24 hours, two (02) liters of water were added, the mixture was mixed again and filtered through a fine cloth. The PIOL biopesticide is now ready. This biopesticide can be preserved by adding a quarter of vegetable oil and transferring the whole into a non-transparent, hermetically sealed can.
2.3. Experimental Design and Treatment
The experimental set-up used was completely randomized and comprised 4 treatments repeated 4 times. A total of 16 mounds were built on July 19, 2022. Each mound was 5 m long, 1 m wide and 40 cm high. The distance between mounds was 1 m. The total surface area of the experimental set-up was 304 m². The treatments applied were:
- CO + WA = 3.20 t/ha of compost + 2.45 t/ha of wood ash;
- CO = 6.40 t/ha of compost;
- WA = 4.90 t/ha of wood ash;
- T0 = Control (no use of fertilizers).
2.4. Crop Management
Crop management consisted in delimiting and setting up the experimental set-up. Soil tillage was carried out by plowing, harrowing and raising of ridges with 40 cm height. Organic fertilizer and/or ash wood was then ploughed into
![]()
Table 1. Fertilizers chemical characteristics.
OM = Organic Matter; C = total Carbon; N = total Nitrogen; P = total Phosphorus; K= total Potassium.
the ridges receiving these treatments. Transplanting of sweet potato cuttings was carried out on July 20th, 2022, with 30 cm spacing on each ridge. There were 15 plants per line per ridge, i.e. a total of 241 plants for the entire system. Two (02) superficial weedings were carried out to reduce the rate of weed growth. PIOL (biopesticide) treatments were also carried out using a pulverizer with a dosage of 01 liter of biopesticide for 9 liters of water. Plants were sprayed every two (02) to three (03) weeks.
2.5. Crop Data Collection
Data collection consisted in evaluating growth and yield parameters at harvest of orange-fleshed sweet potato. Growth parameters include plant height (HP), diameter at the base of the main stem collar (DP) and number of branches per plant (NB). HP was measured in cm using a metric tape, from the base of the main stem to its terminal bud. DP was measured with a caliper at the base of the main stem collar. NB was measured by counting the number of branches from the main stem. These measurements concerned all plants on each hillock. Yield parameters were total number of total tubers (NTT), number of small tubers (NST), number of large tubers (NLT) and tubers yield (TY) per treatment. Tubers were then separated into 2 batches of large and small tubers and counted. A tuber was considered small if it measured less than 10 cm in length and weighed less than 200 g. Tuber yield was determined by weighing the tubers per treatment in kg/m2 and extrapolating to t/ha.
2.6. Soil Sampling and Analysis
Soil samples were taken at the sweet potato harvest. Five (05) elementary soil samples were performed in the depths of 0 - 40 cm from the rhizosphere of 5 plants according to each treatment or ridge. A composite sample was then formed for each treatment by collecting and mixing 100 g of soil from the five (05) elementary samples per treatment. A total of four (04) soil samples corresponding to the four (04) treatments were analyzed. These soil samples were air-dried and sieved with a two (02) mm mesh for analysis of the following chemical parameters in the < 2 mm fraction: total soil organic matter (OM) and carbon (C) in % by the dry combustion method [35] ; total nitrogen (N) in % by the Kjeldahl method [36] ; total phosphorus (P) in % by the Bray I method [37] ; total potassium (K) in % by the flame photometry method [38] ; pH (H2O 1:2.5 suspension (p/v)) and pH (KCl 1:2.5 suspension (p/v)) by the AFNOR method [39] .
2.7. Statistical Analysis
EXCEL 2019 was used for data input. The Shapiro-Wilk test was used to test the normality of the data collected. For each of the orange-fleshed sweet potato parameters evaluated, a comparison of means as a function of treatments was carried out using analysis of variance (ANOVA) and a TukeyHSD test of separation of means at the 5% threshold. Principal component analysis (PCA) was performed to show the relationship between growth parameters, sweet potato harvest yields and soil parameters, as well as their interactivity with treatments, using Pearson’s correlation at the 5% threshold. XLSTAT 4.1, 2023 1.1 (1398) ADDINSOFT software was used for these analyses.
3. Results and Discussion
3.1. Results
3.1.1. Effect of Treatments on Orange-Fleshed Sweet Potato Growth Parameters
Table 2 shows the effect of treatments on average plant heights and diameters and the number of sweet potato plant branches as a function of treatment. Analysis of the table shows that mean values for plant heights (HP) ranged from 48.64 ± 2.02 cm under WA to 62.87 ± 2.33 cm under CO treatment; for diameters at the base of the plant collar (DP) from 1.00 ± 2.02 cm under WA to 1.13 ± 0.02 cm under CO. Mean numbers of branches from the main stem (NB) ranged from 4.92 ± 0.04 under TA to 5.04 ± 0.05 cm under CO. ANOVA at the 5% threshold shows that heights and plants were significantly improved (P < 0.001) according to treatments. The TukeyHSD mean separation test at the 5% threshold shows that the CO treatment significantly increased plant height by 16% compared to the WA treatment, and plant diameter by 12% and 7% compared to the WA and T0 treatments respectively. The CO treatment significantly increased average plant height by 10% compared to the WA treatment.
![]()
Table 2. Effect of treatments on plant growth parameters.
CO + WA = 3.20 t/ha of compost + 2.45 of t/ha wood ash; WA = 4.90 t/ha of wood ash; CO = 6.40 t/ha of compost; T0 = Control; HP = Plant heights; DP = Plant diameter from base of main stem per plant; NB = Number of branches per plant. P = Probability according to ANOVA at the 5% threshold. Means ± standard errors of the same column with the same letter do not differ significantly according to the TukeyHSD test at the 5% threshold. P ≤ 0.001 (HS) = highly significant; P ≥ 0.05 = not significant (NS).
3.1.2. Effect of Treatments on Yield Parameters of Orange-Fleshed Sweet Potato at Harvest
The effect of treatments on mean values per hectare of orange-fleshed sweet potato yield parameters is recorded in Table 3. Total number of tubers (NTT) ranged from 4132 ± 277 under the T0 treatment to 5625 ± 240 under the CO +
WA treatment. Numbers of small tubers (NST) ranged from 2604 ± 160 under CO to 2240 ± 160 under WA. Numbers of large tubers (NLT) ranged from 1563 ± 286 under TA to 3151 ± 248 under CO + WA. Tuber yields (TY) ranged from 6.98 ± 0.70 under T0 to 10.16 ± 0.61 t/ha under CO + WA Treatments significantly and differently affected total and large tuber numbers and tuber yields (ANOVA, 5% threshold). The CO + WA treatment significantly improved total tuber numbers, large tuber numbers and tuber yields by 27 (P ≤ 0.01); 50 (P ≤ 0.01) and 31% (P < 0.05) respectively, compared with the control T0 (TukeyHSD, 5% threshold). Also, the WA treatment significantly favored the number of large tubers by 43%, compared with T0 (P ≤ 0.01).
![]()
Table 3. Effects of treatments on sweet potato harvest yield parameters.
CO + WA = 3.20 t/ha of compost + 2.45 of t/ha wood ash; WA = 4.90 t/ha of wood ash; CO = 6.40 t/ha of compost; T0 = Control; NST = Number of small tubers; NLT = Number of large tubers; TY = tubers yield. Numbers are means ± standard errors of evaluated parameters. P = Probability according to ANOVA at the 5% threshold. Means ± standard errors of the same column with the same letter do not differ significantly according to the TukeyHSD test at the 5% threshold. P < 0.05 = significant (S); P ≥ 0.05 = not significant (NS).
3.1.3. Effect of Treatments on Residual Soil Fertility under Orange-Fleshed Sweet Potato Cropping
Table 4 shows the effects of treatments on residual soil fertility. Residual soil parameter values varied: OM content varied from 1.29% under WA to 0.85% under T0; N content from 0.07% under CO + WA to 0.04% under T0; total P and K contents from 0.09% and 0.18% respectively under WA to 0.07% and 0.09% under T0. The C/N ratio varied from 10 under CO + WA and 12 under CO + WA; CO; WA and T0. pHH2O and KCl varied by 6.88 and 5.67 respectively under WA versus 5.68 and 4.96 under T0. The CO + WA treatment increased MO, N, P, K, pH2O and pHKCl contents by 26%; 38%; 1%; 39%; 8%; and 3% respectively, compared with those recorded under T0. The C/N ratio under CO + WA was reduced by 20% compared with the T0 treatment. MO, N, P, K, pH2O and pHKCl contents improved by 15%; 17%; 11%; 16%; 10%; 11% under CO and 34%; 34%; 16%; 50%; 17%; 13% under WA, respectively.
![]()
Table 4. Effect of treatments on soil residual mineral content.
CO + WA = 3.20 t/ha of compost + 2.45 of t/ha wood ash; WA = 4.90 t/ha of wood ash; CO = 6.40 t/ha of compost; T0 = Control; OM = Organic Matter; C = total Carbon; N = total Nitrogen; P = total Phosphorus; K= total Potassium.
3.1.4. Principal Component Analysis Growth, Yield-Traits and Yield of Orange-Fleshed Potato and Soil Parameters
Figure 2 shows the principal component analysis (PCA) of orange-fleshed sweet potato growth, yield and soil parameters. Treatments were projected as active observations. The analysis of variance shows that 82.85% of the variables contribute to the formation of the 2 axes. Variables such as number of total tubers (NTT), number of large tubers (NLT) and tuber yields (TY) contributed strongly
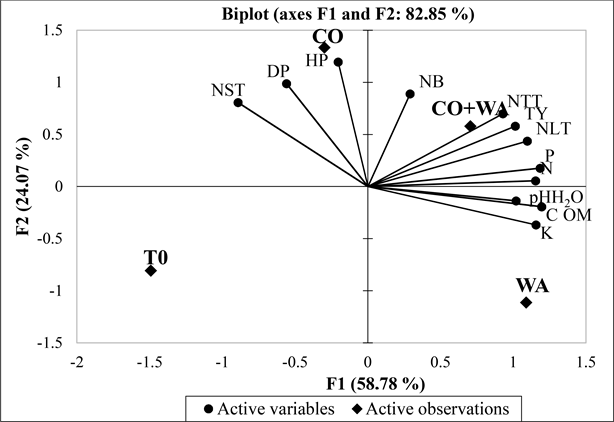
CO + WA = 3.20 t/ha of compost + 2.45 of t/ha wood ash; WA = 4.90 t/ha of wood ash; CO = 6.40 t/ha of compost; T0 = Control; HP = Plant heights; DP = Plant diameter from base of main stem per plant; NR = Number of branches per plant; NTT = number of total tubers; NST = Number of small tubers; NLT = Number of large tubers; TY = tubers yield; OM = total organic matter (%); C: total carbon; N = total nitrogen; P = total phosphorus; K = total potassium.
Figure 2. Principal component analysis of sweet potato growth and harvest yield parameters.
to the formation of axis 1, with R2 values of 0.96; 0.99; 0.99 respectively. Soil content variables for OM, C, N, P, K and soil pH20 are significantly correlated with this axis, with respective R2 values of 0.97; 0.97; 0.90; 0.95; 0.91; 0.71. Axis 2 is formed by the variables plant height (HP) and diameter (DP), number of branches (NB) and number of small tubers (NPT), with R2 values of 0.87; 0.97; 0.47 and 0.75 respectively. The CO + WA treatment is positively correlated with axis 1 (R2 = 0.56), i.e. with yield parameters (NTT, NLT and TY) and soil contents of OM, C, N, P, K and pH 20, and is opposed to the T0 treatment (R2 = −0.91). On the other hand, the CO treatment was positively correlated with axis 2 (R2 = 0.861), i.e. with growth parameters (HP, DP and NB), and opposed to the WA treatment (R2 = −0.65). PCA shows that the 2 axes discriminate between growth parameters associated with the CO treatment and yield and soil parameters associated with the CO + WA treatment.
3.2. Discussion
The aim of the present study was to evaluate the effects of agro-ecological practices on orange-fleshed sweet potato yield performance and residual soil fertility. Thus, the effects of application of 3.20 t/ha of compost + 2.45 t/ha of wood ash (CO + WA); 4.90 t/ha of wood ash (WA); 6.40 t/ha of compost (CO) and control with no inputs (T0) on growth, yields-trait and yields of orange-fleshed sweet potato and residual soil fertility were evaluated. Results showed that compost treatments significantly increased plant heights compared with wood ash and absolute control treatments; and plant diameters compared with compost + wood ash, wood ash and control treatments. These observations are similar of those of Akpaninyang et al. [40] , who showed that the application of poultry manure alone led to an increase in the leaf area index and above-ground biomass production of sweet potatoes. Contrary the application 10 t/ha compost did not significantly improve the plant height of potato (Solanum tuberosum L.) compared to the control in Western Ethiopia [41] . The effects of the compost on the growth of orange-fleshed sweet potato would thus be linked to the additional inputs of 52.18% OM; 1.81% N; 1.2% P; and 2.05% K from the compost, but also to its indirect effect on the physical, chemical and biological properties of the soil. Indeed, the organic matter contained in compost increases the soil’s ability to buffer pH changes, improves cation exchange capacity (CEC), reduces phosphate fixation and acts as a reservoir for secondary nutrients and micronutrients [42] [43] . The pH2O and pHKCl of soil under compost treatment was improved by 10% and 11%, respectively, compared to control. Thus, plant growth being strongly affected by pH changes in the soil, mainly due to changes in plant nutrient availability [44] , could explain the growth of plants under compost application. Also, the micro-organisms and microbial processes involved in soil biological processes are strongly affected by soil pH [45] . In general, an increase in pH will stimulate plant growth in acid soils, as the optimum pH for most plants is around 6.5 - 7, because nutrients such as P and K in particular are more available at a neutral pH [46] . Microbial activity will facilitate the mineralization of nutrients (e.g. inorganic nitrogen) [47] [48] . By stimulating biological activities and increasing soil humus via the decomposition of compost, microorganisms improve soil structure and mineral fertility. This promoting the uptake of major nutrients such as N, P and K by sweet potato plants for vegetative growth evidenced by the high pH values following organic inputs.
At the same time, the application of compost + wood ash to the soil favored the growth of orange-fleshed sweet potato plants compared with the application of wood ash alone. The addition of ash to the compost would therefore have accelerated the mineralization of the compost to release nutrients, notably N, for the vegetative growth of orange-fleshed sweet potato plants. In fact, the application of wood ash also leads to increased microbial mineralization of soil organic nitrogen into plant-available nitrogen in the form of
and
[47] [48] [49] . Otherwise, the application of 4 t/ha of wood ash, 2 t/ha wood ash + 5 t/ha of compost did not significantly improve the plant height of the potato compared with the control in Westhern Ethiopia [41] .
Total tuber, large tuber numbers and tuber yields were significantly affected under the compost + wood ash treatment compared with the control treatment. This would be due to the fact that the combined compost and wood ash inputs added a total of 81.35% OM; 50.19% C; 3.17% N; 2.31% P; 16.22% K to the initial soil contents of these elements, which improved residual soil contents by 26% MO; 38% N; 13% P; 39% K; 8% pH2O; and 3% pHKCl compared with the control. These inputs thus improved soil fertility to release the mineral elements needed to promote tuberization and sweet potato yields. The positive effects of wood ash application on tree production had also been demonstrated on sites that receive nitrogen deposition or are rich in organic matter, such as peat soils, whereas ash does not improve tree production on mineral soils where nitrogen is the limiting nutrient [50] [51] . In nitrogen-limited systems, the combined application of ash and nitrogen can be a solution to the imbalance in nutrient content of wood ash [52] [53] . Thus, organic fertilization combined with ash had the effect of improving the soil’s mineral pool, making minerals available to plants and positively affecting crop yields. Contrary to this research result, the application of 2 t/ha of wood ash + 5 t/ha of compost did not significantly total number and yields of tubers of the potato compared with the control in Western Ethiopia [41] . However, application of 4 t/ha wood ash and 10 t/ha compost alone significantly increased the percentage of number of small tubers compared with the control and 2 t/ha of wood ash + 5 t/ha of compost. Furthermore, the wood ash treatment significantly promoted a 43% gain in the number of large tubers of orange-fleshed sweet potato compared with the absolute control. This means that the application of wood ash containing 29.17% MO; 1.36% N; 1.11% P; 14.17% K with residual soil contents of 1.29% MO; 0.06% N; 0.09% P; and 0.18% K favoured a gain in the number of large tubers to the detriment of vegetative plant growth and overall tuber yield. The application of 4 t/ha of wood ash significantly increased the percentage of number of large tubers, in contrast to control in Ethiopia [41] . Also, previous results had shown that wood ash application does not impact total tuber number as a yield component [47] , but impacts tuber dry matter content, which contributes to tuber yield [54] . Indeed, the application of ash activates a number of enzymes involved in photosynthesis, carbohydrate metabolism and aids in the translocation of carbohydrates from leaves to storage roots [55] . Potassium (K) in wood ash increases tuber size, not number [56] . In this sense, Trehan and Grewal [57] had previously observed that, in general, fast-growing potato varieties producing large tubers respond more to K than small tuber varieties, as the application of K is known to increase tuber size. K is also vital for the production and movement of carbohydrates to tubers, supports photosynthesis and protein synthesis, regulates stomatal opening, increases nitrogen utilization, promotes assimilate transport, improves plant stress resistance, water use efficiency and plant enzyme activation [58] [59] . Furthermore, the potassium contained in wood ash promotes tuberized root development, enhances resistance to disease and increases vitamin A production [60] .
Compost + wood ash, wood ash and compost increased OM, N, P and K contents, and raised pHH2O and pHKCl compared with the control. These various increases are attributable to the amending power of compost and ash, reflecting their effectiveness in improving residual soil fertility. The compost + wood ash treatment performed better, improving residual soil N content by 38%, while the Wood ash treatment increased soil K content by 50% compared with the control. Soil K content under compost + wood ash improved by 39%, and soil N content by 34% under wood ash. The increase in N content under compost + wood ash is attributable to the availability of this element in both compost and ash (1.81% for compost and 1.36% for wood ash). In addition, wood ash would have a catalytic effect, accelerating the degradation of OM contained in these 2 substrates (52.18% for compost and 29.17% for wood ash), in order to release N into the soil. This is justified by the reduction of the 20% of C/N ratio under compost + wood ash compared with the other treatments. The increasing of 50% of residual K content of soil under wood ash treatment is not unexpected, since the ash used for K contains 14.17% K, compared with 2.05% for compost. In addition, the improvement of 1.2 unit of pHH2O of soil under wood ash treatment compared with the control indicates to the neutralization capacity induced by wood ash used as a soil amendment. In this sense, An and Park [61] had demonstrated the effects of wood ash improvement on soil pH, organic matter, available P, exchangeable bases (K+, Na+, Ca2+ and Mg2+) and CEC of a saline landfill soil, an infertile forest soil and an acid forest soil, with a predominance of improvement and neutralization in the acid forest soil. Also, fly ash doses of 0, 50, 100 and 150 t/ha increased pH from 6.66 to 7.30; total P from 31.74 - 52.21 mg/100g; and total K from 16.19 - 25.75 mg/100g on ultisols in Indonesia under growing water spinach [62] . The improvement in soil parameters under compost-based treatment is in line with that obtained by Wilson et al. [63] . The latter had found that compost application increased soil pH and nutrient concentrations (K, Ca, Mg and S), reduced potato mound bulk density by 8%, increased soil organic carbon by 24% as well as soil respiration.
The compost + wood ash treatment improved yield trait and yields of orange-fleshed sweet potato and the residual soil fertility at harvest, while the compost treatment promoted the vegetative growth of sweet potato plants, according to the results of principal component analysis (PCA). Comparative research had shown that bulk density, porosity, water content and soil chemical properties (pH, C, N, P, K, Ca and Mg) significantly influenced sweet potato tuber yield in the Alfisols and therefore dictated their performance in southwestern Nigerian [64] . Indeed, Silva et al. [65] had found that the interaction between nitrogen and potassium, despite antagonistic effects on leaf P and starch accumulation, increased productivity and average commercial tuber mass and yam profitability. However, in fertilization management, the most common nitrogen-related interactions are with potassium, and these nutrients are absorbed in relatively high proportions and have non-competitive associations, where nitrogen favors support of vegetative growth and potassium favors tuber formation during the yam development cycle [66] . Furthermore, Asfaw [41] had shown that marketable and total potato tuber yield was positively and significantly correlated with growth components such as plant height, total fresh biomass and number of stems. According to this author, marketable tuber yield was positively and significantly correlated with yield components such as the total number of tubers per plant and the number of marketable potato tubers, but negatively and significantly correlated with the number of non-marketable tubers.
4. Conclusion
Evaluation of the effects of ecological practices on the yield performance of the JEWEL variety of orange-fleshed sweet potato showed that inputs of 6.40 t/ha of compost were effective in improving plant growth parameters, while combined inputs of compost and wood ash increased harvest yields. Remarkably, the compost treatment used alone, favored vegetative plant growth whereas the wood ash application increased the number of large tubers. However, compared to the absolute control, all treatments improved residual soil fertility. The combined of the compost and wood ash application improved residual N fertility as a result of the reduction in the soil’s C/N ratio. On the other hand, the application of wood ash alone was more effective in increasing residual potassium content and neutralization soil pH. Compost + wood ash improves yields of the JEWEL variety of orange-fleshed sweet potato and residual soil fertility, unlike compost application, which promotes vegetative plant growth. In the interests of agronomic extension, further investigations are necessary to determine the treatment would promote good tuber preservation and storage and assess the technico-economic profitability of orange-fleshed sweet potato production.
Acknowledgements
We would like to thank the Non-Governmental Organization Association pour la Recherche et la Formation en Agro-écologie (ARFA) and all ARFA staff for their material, moral and financial support in carrying out this research.