Histopathological and Immunohistochemical Study of Peri-Implant Epithelium ()
1. Introduction
After implant placement, an implant-epithelial interface is formed over the course of wound healing. However, since the covering epithelium that protects the inside communicates with the external environment, there is always a risk of inflammation. Peri-implant disease affects the tissues around implants and has an inflammatory origin. Despite the success rates of dental implants, peri-implant disease, which consists of two main conditions, peri-implant mucositis and peri-implantitis, is the most common complication in implant dentistry [1] . Peri-implant disease has a high-prevalence; the mean prevalences of peri-implant mucositis and peri-implantitis were 52% and 18%, respectively [1] [2] .
The oral mucosa around a dental implant is covered by peri-implant epithelium (inner implant epithelium facing the implant and outer implant epithelium facing the oral cavity) [3] , and several studies of their characteristics when healthy have been reported [3] [4] [5] [6] , but studies of the inner implant epithelium in peri-implantitis are very few [7] , and there has been insufficient investigation of the relationship with the mechanism of inflammation.
Oral epithelium consists of three protective barriers: a mechanical barrier, innate immunity, and adaptive immunity [8] . Defensins, a family of antimicrobial peptides, are vital contributors to the innate immune response due to their ability to recognize and neutralize invading microorganisms [8] [9] . In particular, human beta-defensins (HBDs) are epithelial-derived antimicrobial peptides. The expression of three HBDs (HBD-1, 2, and 3) has been reported in oral mucosa and gingiva [10] , and they contribute to gingival health and periodontal disease [11] . Furthermore, it has been reported that increased HBD‑3 expression in periodontal tissue may contribute to periodontal regeneration [9] [12] . However, the HBD expression patterns of inner implant epithelium as protection against oral infections, especially peri-implant disease, are not well understood [11] .
To investigate the histopathological features of the inner implant epithelium in peri-implantitis, the inner marginal epithelium of periodontitis and the inner implant epithelium were compared using histological and immunohistochemical methods.
2. Materials and Methods
2.1. Subjects
The characteristics of the subjects in this study are summarized in Table 1.
![]()
Table 1. Clinical and histopathological information for the study subjects.
There were 10 cases of peri-implantitis (3 male, 7 female, 69.5 ± 8.6 years), 11 cases of periodontitis (7 male, 4 female, 67.4 ± 12.40 years), and 10 controls (5 male, 5 female, 52.3 ± 14.6 years). Patients with periodontitis, peri-implantitis, and epulis as a control were selected from the pathology files of the Department of Pathology, Nihon University School of Dentistry at Matsudo. The diagnostic criteria for peri-implant disease were based on the sixth European Workshop on Periodontology (EWP) [13] , the American Academy of Periodontology, and the eighth EWP [14] [15] . Only cases in which the peri-implant (inner and outer) epithelium was collected histologically for use in histopathological and immunohistochemical investigations were included. The inner marginal epithelium attached to the epulis resection specimens of 10 cases was used as the control.
Included in this study were cases clinically diagnosed and biopsied by certified physicians or specialists of the Japanese Society of Oral and Maxillofacial Surgeons or Japanese Society of Oral Implantology. Excluded were patients with other specific inflammatory diseases including autoimmune diseases, oral mucosal diseases, malignant tumors, and those currently taking various preparations or medicines those may induce periodontal diseases.
2.2. Histopathological and Immunohistochemical Examination
One set of sections was stained with hematoxylin and eosin (HE) using standard staining protocols. After HE observation, the thickness of the inner implant/marginal epithelium in each case was observed and photographed under high-power (×200) observation with an optical microscope (BX51, Olympus, Japan) and randomly measured using Win Roof version 3.4 image analysis software (Image J, NIH, Bethesda, MD, USA) in 5 fields.
Immunohistochemical studies were conducted using 10% neutral formalin solution-fixed, paraffin-embedded tissues from all cases. Several serial 4-µm-thick sections were prepared and deparaffinized in xylene and hydrated in graded ethanol solution. Ki-67 antigen (MIB-1, 1:100, Dako, Glostrup, Denmark) and HBD-3 antigen (1:200, Novus Biologicals, Littleton, CO, USA) were used to evaluate cell proliferative ability and biological defense mechanisms of the epithelium, respectively. After antigen retrieval (Ki-67, 10 mM Tris-EDTA buffer, pH 9.0, 40 min; HBD-3, 10 mM citrate buffer, pH 6.0, 20 min) and blocking (3% H2O2, 10 min), the slides were incubated with Ki-67 for 60 min and HBD-3 overnight at 4˚C. After the EnVision + Polymer System (Dako EnVision+ Dual Link System-HRP, Dako) was used for 60 min, it was developed in a solution of 3,3'-dianibobenzidine tetrahydrochloride (Dako Liquid DAB+ Substrate Chromogen System, Dako). Finally, all sections were counterstained with Mayer’s hematoxylin for 3 min. The procedures were conducted while confirming the absence of technical false positives and false negatives.
Ki-67 and HBD-3 induced dark brown reactions in epithelial cell nuclei and cytoplasm, respectively. For semi-quantitative evaluation, Ki-67 was evaluated by the positivity rate (number of positive cells/total number of cells). For HBD-3, staining intensity was graded as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong); the stained region was scored as 0 (negative), 1 (<10%), 2 (11% - 50%), 3 (51% - 80%), and 4 (>80%). The two scores were multiplied, and the intensity and region score (IRS, value from 0 - 12) was determined [16] . The measurements were performed in 10 hot-spot areas by two oral pathologists and a dentist, and the means of these 10 measurements were recorded.
2.3. Statistical Analysis
The SPSS 27.0 software package (IBM Inc., Armonk, NY, USA) was used to perform statistical analysis. To compare the lengths of epithelial legs, the Mann-Whitney U test was used. In the comparisons of average Ki-67 positivity rates for within the group of epithelial cell types were Wilcoxon signed-rank test, and inter-group of that were Kruskal-Wallis test and Steel-Dwass test used, respectively. As for HBD-3 for staining attitude, comparisons within the group of epithelial cell types were Friedman test and Bonferroni correction, and inter-group of that were the Kruskal-Wallis test and Steel-Dwass test used. A significance level of p < 0.05 was considered significant.
2.4. Compliance with Ethical Standards
Informed consent was obtained from all individuals included in the study. All procedures in studies involving human participants were conducted in accordance with the ethical standards of the Committee on Studies Involving Human Beings of Nihon University School of Dentistry at Matsudo (EC21-008A) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
3. Results
3.1. Histopathological Findings
1) Histopathological findings of the inner (marginal/implant) epithelium
Representative HE-stained features of the inner epithelium are shown in Figure 1. In periodontitis, the epithelial ridges extended irregularly, and a high degree of inflammatory cell infiltration was observed in the subepithelial connective tissue (Figure 1(a), Figure 1(b)). In peri-implantitis, although the inner implant epithelium was thin, it was slightly extended in some parts, and moderate infiltration of inflammatory cells was observed in the subepithelial connective tissue (Figure 1(c)). The superficial layer of the inner implant epithelium showed parakeratosis, and detachment of the keratin fragments was observed (Figure 1(d)). In the control, the inner marginal epithelium was non-keratinized stratified squamous epithelium that extended slightly in a bud shape (Figure 1(e)). The basal cells were columnar-shaped and arranged with a slight palisading pattern (Figure 1(f)).
2) Measurement results of inner (marginal/implant) epithelium thickness
It was significantly higher in periodontitis (156.2 [138.0, 186.4] µm) than in peri-implantitis and control, 70.7 [67.5, 97.5] µm and 80.7 [76.6, 89.4] µm, respectively (p < 0.001).
3.2. Immunohistochemical Evaluation
Representative features on immunohistochemical staining with Ki-67 and HBD-3 in the inner/outer marginal epithelium of periodontitis and control, and inner/outer implant epithelium of peri-implantitis are shown in Figure 2.
1) Ki-67 staining findings
With Ki-67 staining, numerous positive reactions were detected throughout all layers of the inner marginal epithelium, whereas positive reactions were observed in the basal or para-basal layer of the outer marginal epithelium in periodontitis (Figure 2(a), Figure 2(b)). In peri-implantitis, positive reactions were detected in the basal or para-basal layer of the inner implant epithelium, whereas scattered positive reactions were observed in the basal layer of the outer implant epithelium (Figure 2(c), Figure 2(d)). In control, positive reactions were
![]()
Figure 1. Representative histopathological features of the inner (marginal/implant) epithelium (hematoxylin and eosin staining, ((a), (c), (e)): ×10, ((b), (d), (f)): ×200). (a) In periodontitis, the epithelial ridges irregularly extend. (b) In periodontitis, neutrophils infiltrate into the epithelium, and congestion was observed just below the basement membrane. (c) In peri-implantitis, the inner epithelium was thin, it was slightly extended in some parts. (d) In peri-implantitis, the superficial layer showed parakeratosis, and detachment of the keratin fragments were observed. (e) In control, the inner epithelium was non-keratinized stratified squamous epithelium and extends slightly in a bud shape. (f) In control, the basal cells were columnar shape and arranged with a slight palisading pattern.
scarcely observed in the inner marginal epithelium, but scattered positive reactions were observed in the basal layer of the outer marginal epithelium (Figure 2(e), Figure 2(f)).
2) HBD-3 staining findings
With HBD-3, the basal layer of the inner marginal epithelium showed a high degree of strong positivity, whereas in the outer marginal epithelium, positive reactions were detected from the basal and para-basal layers to the lower layers of the spinous layer in periodontitis (Figure 2(g), Figure 2(h)). In peri-implantitis, there was diffuse weakly positive staining throughout all layers in the inner implant epithelium, with weak staining in the parabasal layer of the
![]()
Figure 2. Representative immunohistochemical features of the inner epithelium ((a), (c), (e)): Ki-67, ((g), (i), (k)): HBD-3) and the outer epithelium ((b), (d), (f)): Ki-67, ((h), (j), (l)): HBD-3, ((a), (c), (e), (g), (i), (k)): ×100, ((b), (d), (f), (h), (j), (l)): ×400). (a) In periodontitis, numerous positive reactions were detected throughout all layers of the inner epithelium. (b) In periodontitis, positive reactions were observed in the basal or para-basal layer of the outer epithelium. (c) In peri-implantitis, positive reactions were detected in the basal or para-basal layer of the inner epithelium. (d) In peri-implantitis, scattered positive reactions were observed in the basal layer of the outer epithelium. (e) In control, positive reactions were hardly observed in the inner epithelium. (f) In control, scattered positive reactions were observed in the basal layer of the outer epithelium. (g) In periodontitis, the basal layer of the inner epithelium showed a high degree of positivity. (h) In periodontitis, positive reactions were detected from the basal and para-basal layers to the lower layers of the spinous layer in the outer epithelium. (i) In peri-implantitis, there was diffusely mild positive staining throughout all layers in the inner epithelium. (j) In peri-implantitis, mild positive staining in the parabasal layer of the outer epithelium. (k) In control, the basal layer of the sulcular epithelium showed mild to moderate positive reactions. (l) In control, positive reactions were detected from the basal and para-basal layers to the entire spinous layer in the outer epithelium.
outer implant epithelium (Figure 2(i), Figure 2(j)). In control, the basal layer of the inner marginal epithelium showed moderate to weak reactions, whereas in the outer marginal epithelium, moderate to strong reactions were detected from the basal and para-basal layers to the entire spinous layer (Figure 2(k), Figure 2(l)).
3) Semi-quantitative evaluation of immunohistochemical staining
The Ki-67 positivity rates, both for inner epithelium and outer epithelium, were significantly higher in the following order: periodontitis, peri-implantitis, and control (p < 0.001, Table 2). In addition, the rate was significantly higher in the outer epithelium than in the inner epithelium in peri-implantitis and control, however, the inner epithelium is significantly elevated in the case of periodontitis (p < 0.001, Figure 3).
Table 3 shows the immunohistochemical attitude of HBD-3. For HBD-3, when comparing the epithelial types within the same disease, significant differences were observed among the three groups (inner epithelium, outer epithelium deep layer, and outer epithelium superficial layer) except for the staining region and IRS of the outer epithelium deep layer (p < 0.001). In particular, the IRS was significantly higher for inner epithelium than for outer epithelium (superficial layer) (p < 0.001) in the three groups. The comparisons of IRS for each epithelial type among the three groups, significantly higher values were seen in inner epithelium of periodontitis than that of peri-implantitis (p < 0.001, Figure 4).
4. Discussion
The inner epithelium facing the enamel or implant consists histologically of
![]()
Table 2. Ki-67 positivity rate of each epithelium of periodontitis, peri-implantitis, and control.
a; Wilcoxon rank sum test.
Table 3. Immunohistochemical attitude of HBD-3.
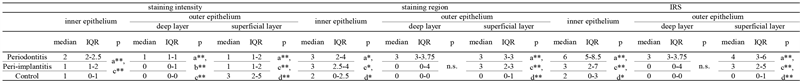
HBD-3; human β defensin-3. IRS: Immunohistochemical intensity and region socre, superficial layer; consisted of para-basal to spinpous layer. a; inner epithelium vs outer epithelium deep layer vsouter epithelium superficial layer, Friedman test; b; inner epithelium vs outer epithelium deep layer, Bonferroni correction; c; inner epithelium vs outer epithelium superficial lyer, Bonferroni correction; d; outer epithelium deep lyer vs outer epithelium superficial layer, Bonferroni correction; *p < 0.05; **p < 0.01; ***p < 0.001. n.s.; not significant; IQR: Interquartile Range.
![]() inner: inner epithelium, outer deep: outer epithelium deep layer, outer super: outer epithelium superficial layer. **: p < 0.001
inner: inner epithelium, outer deep: outer epithelium deep layer, outer super: outer epithelium superficial layer. **: p < 0.001
Figure 3. The immunohistochemical positive rates for Ki-67. The rate was significantly higher in the outer epithelium than in the inner epithelium in peri-implantitis and control, however, the inner epithelium is significantly elevated in the case of periodontitis (p < 0.001).
![]() inner: inner epithelium, outer deep: outer epithelium deep layer, outer super: outer epithelium superficial layer. **: p < 0.001
inner: inner epithelium, outer deep: outer epithelium deep layer, outer super: outer epithelium superficial layer. **: p < 0.001
Figure 4. The immunohistochemical intensity and region score (IRS) of HBD-3. The comparisons of IRS for each epithelial type among the three groups, significantly higher values were seen in inner epithelium of periodontitis than that of peri-implantitis (p < 0.001).
sulcular epithelium (SE) and junctional epithelium (JE) [17] . In humans, the inner epithelium is clearly observed when there is a pathological periodontal pocket [7] . In this study, due to the difficulty distinguishing between SE and JE based on inflammatory modifications, both were collectively observed and compared as the “inner epithelium”, and with periodontitis they were thicker. Turnover of the inner epithelium is activated during the process of repair of chronic inflammation [18] . The thickness of the peri-implant epithelium was 52% of that in periodontitis. Based on the results, the inner implant epithelium appears to show weaker hyperplastic changes during the repair process than the inner epithelium around enamel.
This conjecture was consistent with the results of Ki-67 staining in the present study. Ki-67 is a cell-cycle-associated antigen present in all non-G0 phases of the cell cycle, and the commercially available MIB-1 clone has been applied widely in the practice of surgical pathology as a proliferative marker or prognostic factor [19] . In the present study, the proliferation rate of peri-implant (inner/outer) epithelium was significantly higher in periodontitis than in peri-implantitis. The JE with rapid turnover [17] originates from the enamel organ and constructs the inner marginal epithelium along with sulcular epithelium, which is derived from the oral epithelium [20] [21] . On the other hand, the inner implant epithelium, which originates from the oral mucosa, has a lower capacity for a proliferative and regenerative mechanism than does the normal inner marginal epithelium [4] [18] .
On the other hand, ultrastructural and immunocytochemical studies showed inferior adhesion and endocytic capacity of the peri-implant epithelium compared to normal JE [3] [7] [22] . Periodontitis is a complex inflammatory disease resulting from the interaction between the dental biofilm and host immune responses. The slow cell turnover and weak adhesion to the implant interface [3] [7] [22] are thought to be due to the fact that peri-implant epithelium has less of a protective function than normal peri-marginal epithelium [18] .
Concerning the innate immune response, HBDs, which are epithelial-derived antimicrobial peptides, may play a key role in susceptibility to periodontitis [9] [12] . Of the HBDs, oral mucosal epithelium mainly produces HBD-1 to HBD-3 [8] . The antibacterial properties of HBD-3 were broader and stronger than those of HBD-1 and HBD-2 [23] [24] . HBD-3 is synthesized by keratinocytes, monocytes, and CD4+ T cells and has a broad spectrum of potent antimicrobial activity against Gram-positive and Gram-negative bacteria [25] , in particular. It regulates wound healing, periodontitis, and carcinogenesis [25] [26] [27] . In the outer marginal epithelium of the present study, HBD-3 was detected predominantly in the basal layer and extended to the spinous layer in periodontitis, which was similar to a previous study [8] [26] [28] . Concerning the inner marginal epithelium, weak, strong, and slight HBD-3 positivity was mainly seen in the basal layer in control, periodontitis, and peri-implantitis, respectively, in the present study. These results for control and periodontitis are consistent with previous reports that showed that, when stimulated by external factors, the expression of HBD-3 in epithelial tissues was markedly increased, thus exerting its innate immune defense function [8] [10] . Specifically, for each epithelial type, periodontitis showed significantly higher values than peri-implantitis in the present study. The results were consistent with Shimono et al.’s findings, which supported the notion that β-defensin in healthy inner marginal epithelium is a consequence of the inflammatory response [18] .
On the other hand, the inner implant epithelium showed very weak expression of HBD-3 even during inflammation in the present study. This result suggests the decreasing responsiveness of the inner implant epithelium to inflammation. In previous reports, HBD-3 was found to have important antibacterial activity, along with a possible role in inducing fibroblast proliferation, promoting periodontal regeneration [9] [12] . Furthermore, epithelial cells and fibroblasts are the predominant cells of periodontal tissues and serve as a first-line defense against periodontopathogens [29] . The little HBS-3 expression in the inner implant epithelium is significant in exploring the pathogenesis of peri-implantitis. Decreased production of HBD-3 in keratinocytes composing the inner implant epithelium may be one of the factors affecting tissue repair in peri-implantitis.
There are some limitations of the present study that must be recognized. There was considerable variation in the methods used for collecting, processing, and analyzing the inner epithelium samples. In addition, the synergistic action with other antimicrobial peptides was not examined. Furthermore, the number of samples in this study was not sufficient.
5. Conclusions
The following could be concluded in the present study:
1) The inner epithelium around the implant exhibits weaker hyperplastic changes during the repair process compared to those in periodontitis.
2) The weak expression of HBD-3 in the inner implant epithelium is implicated in the pathogenesis of peri-implantitis.
Acknowledgements
The authors would like to express their sincere gratitude to Mr. Masayuki Ukigaya for his valuable technical guidance in the preparation of pathological tissue specimens and immunohistochemical staining. This work was supported by JSPS KAKENHI Grant Number 22K12850.